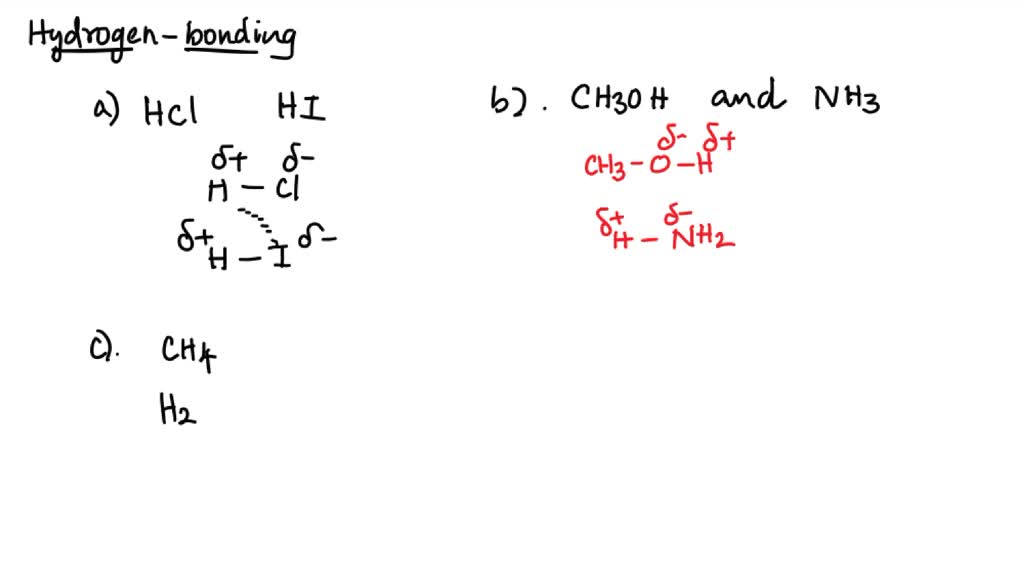
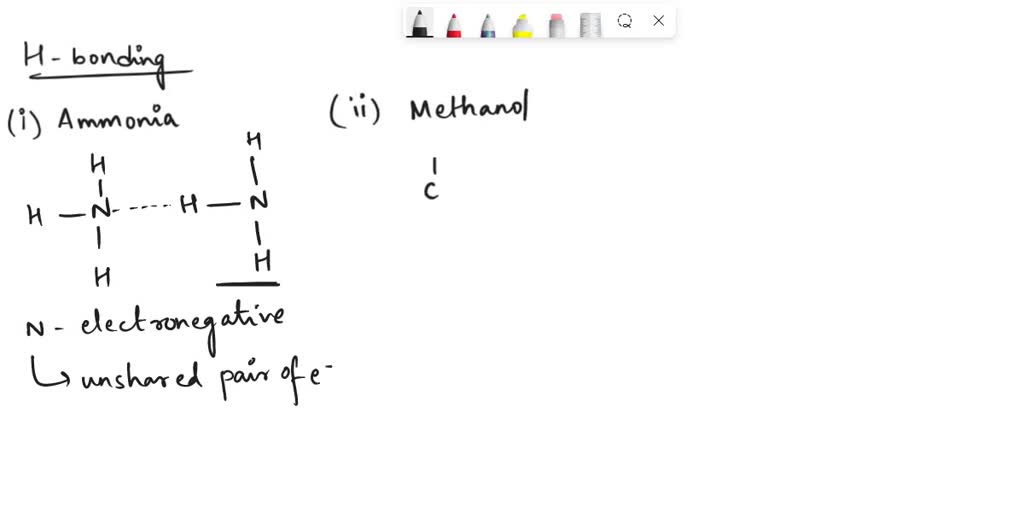
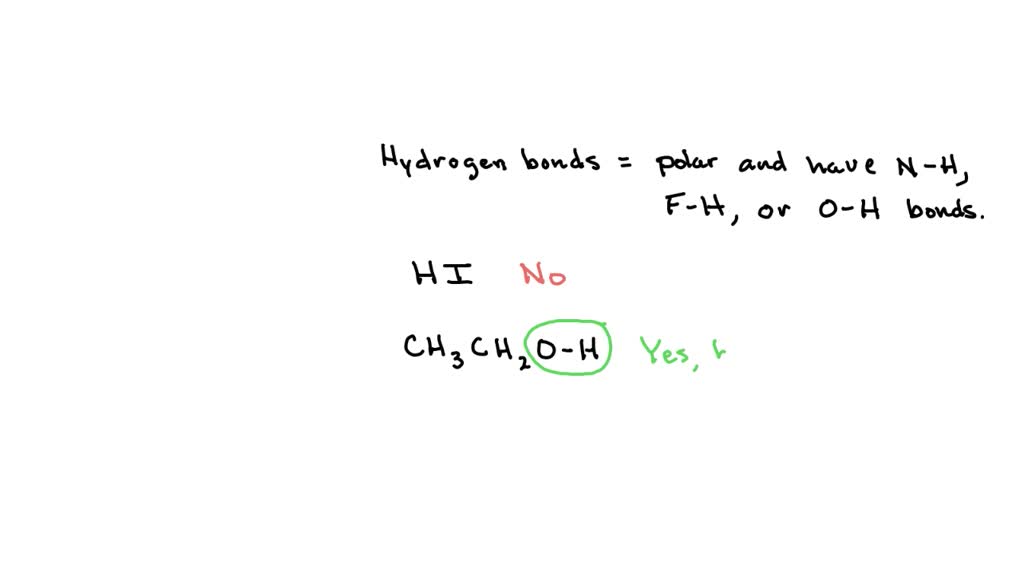
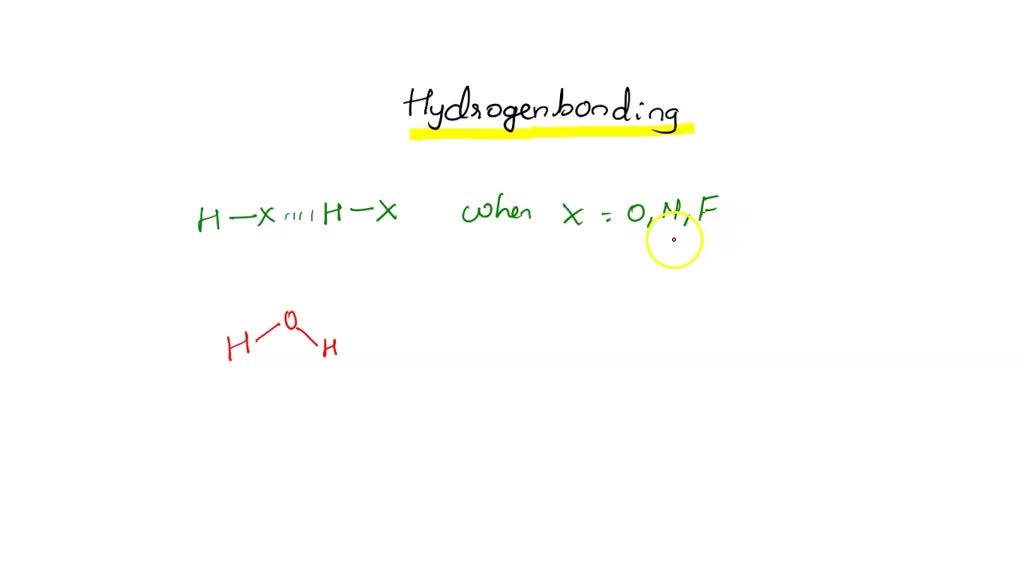
Can Ch3Oh Form Hydrogen Bonds - In principle, the oxygen atom of ch3oh has two unused doublets. Ch₃br has no n, o, or f. It can form hydrogen bonds with other ch₃oh molecules. Each doublet can attract one h atom from foreign molecules. Ch3oh can form hydrogen bonds. So, can ch3oh form hydrogen bonds? In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. The answer is yes, but with some limitations.
Each doublet can attract one h atom from foreign molecules. In principle, the oxygen atom of ch3oh has two unused doublets. The answer is yes, but with some limitations. So, can ch3oh form hydrogen bonds? Ch₃br has no n, o, or f. It can form hydrogen bonds with other ch₃oh molecules. Ch3oh can form hydrogen bonds. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3.
Ch₃br has no n, o, or f. So, can ch3oh form hydrogen bonds? It can form hydrogen bonds with other ch₃oh molecules. Ch3oh can form hydrogen bonds. The answer is yes, but with some limitations. Each doublet can attract one h atom from foreign molecules. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. In principle, the oxygen atom of ch3oh has two unused doublets.
Can Ch3oh Form Hydrogen Bonds Form example download
It can form hydrogen bonds with other ch₃oh molecules. Each doublet can attract one h atom from foreign molecules. The answer is yes, but with some limitations. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. Ch3oh can form hydrogen bonds.
SOLVED Which of the following compounds can form hydrogen bonds with
It can form hydrogen bonds with other ch₃oh molecules. Ch₃br has no n, o, or f. Ch3oh can form hydrogen bonds. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. Each doublet can attract one h atom from foreign molecules.
Oxygen Covalent Bond Diagram
Each doublet can attract one h atom from foreign molecules. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. Ch3oh can form hydrogen bonds. Ch₃br has no n, o, or f. The answer is yes, but with some limitations.
SOLVED a) Circle the molecules below that can form hydrogen bonds with
It can form hydrogen bonds with other ch₃oh molecules. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. Ch₃br has no n, o, or f. In principle, the oxygen atom of ch3oh has two unused doublets. Each doublet can attract one h atom from foreign molecules.
SOLVED 84 2 points Which pairs of compounds can hydrogen bond to each
The answer is yes, but with some limitations. Each doublet can attract one h atom from foreign molecules. In principle, the oxygen atom of ch3oh has two unused doublets. Ch3oh can form hydrogen bonds. It can form hydrogen bonds with other ch₃oh molecules.
SOLVED Which of the molecules can form a hydrogen bond with a water
The answer is yes, but with some limitations. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. In principle, the oxygen atom of ch3oh has two unused doublets. So, can ch3oh form hydrogen bonds? Ch3oh can form hydrogen bonds.
SOLVED 11. What types of secondary bonds can the following structure
Ch3oh can form hydrogen bonds. The answer is yes, but with some limitations. It can form hydrogen bonds with other ch₃oh molecules. So, can ch3oh form hydrogen bonds? In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3.
SOLVED Intermolecular Forces Substances Intermolecular Forces 1
So, can ch3oh form hydrogen bonds? The answer is yes, but with some limitations. Each doublet can attract one h atom from foreign molecules. Ch₃br has no n, o, or f. Ch3oh can form hydrogen bonds.
SOLVED In which case will the two molecules be involved in dipole
The answer is yes, but with some limitations. Each doublet can attract one h atom from foreign molecules. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3. Ch3oh can form hydrogen bonds. Ch₃br has no n, o, or f.
SOLVED Which of the following can form hydrogen bonds? CHA CH3OH CH3F
Ch3oh can form hydrogen bonds. In principle, the oxygen atom of ch3oh has two unused doublets. The answer is yes, but with some limitations. It can form hydrogen bonds with other ch₃oh molecules. So, can ch3oh form hydrogen bonds?
Each Doublet Can Attract One H Atom From Foreign Molecules.
Ch3oh can form hydrogen bonds. It can form hydrogen bonds with other ch₃oh molecules. Ch₃br has no n, o, or f. In principle, the oxygen atom of ch3oh has two unused doublets.
So, Can Ch3Oh Form Hydrogen Bonds?
The answer is yes, but with some limitations. In summary, the molecules that can form hydrogen bonds with each other are nh3, h2o, ch3oh, and ch3och3.