Can Metals Form Covalent Bonds - Metal do form covalent bond. So, can metals form covalent bonds? The answer is yes, but under specific conditions. Having established that there is no real difference between coordinate bonds and covalent bonds, the only real question is how. However, it is not the way. It is very common in transition metal like platinum, palladium. Metals typically do not form covalent bonds. They usually form ionic bonds with nonmetals. Metallic bonds exist in metal crystal lattices. But in other compounds containing a rwo or a few metal atoms, they can be covalently bonded.
Metal do form covalent bond. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. They usually form ionic bonds with nonmetals. The answer is yes, but under specific conditions. Metals typically have a high number of valence. It is very common in transition metal like platinum, palladium. Metallic bonds exist in metal crystal lattices. Metals typically do not form covalent bonds. But in other compounds containing a rwo or a few metal atoms, they can be covalently bonded. Having established that there is no real difference between coordinate bonds and covalent bonds, the only real question is how.
Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Having established that there is no real difference between coordinate bonds and covalent bonds, the only real question is how. Metals typically do not form covalent bonds. Metallic bonds exist in metal crystal lattices. They usually form ionic bonds with nonmetals. But in other compounds containing a rwo or a few metal atoms, they can be covalently bonded. Metals typically have a high number of valence. It is very common in transition metal like platinum, palladium. So, can metals form covalent bonds? The answer is yes, but under specific conditions.
Online Essay Help amazonia.fiocruz.br
Metals typically do not form covalent bonds. Metals typically have a high number of valence. The answer is yes, but under specific conditions. They usually form ionic bonds with nonmetals. Metal do form covalent bond.
Metallic Bonding Definition and Properties
Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. However, it is not the way. They usually form ionic bonds with nonmetals. Having established that there is no real difference between coordinate bonds and covalent bonds, the only real question is how. The answer is yes, but under specific conditions.
What Happens When Two Nitrogen Atoms Share Electrons MarisolkruwLee
It is very common in transition metal like platinum, palladium. Metals typically do not form covalent bonds. The answer is yes, but under specific conditions. However, it is not the way. Metal do form covalent bond.
Periodic Table Groups Definition And Example
The answer is yes, but under specific conditions. Metals typically do not form covalent bonds. Having established that there is no real difference between coordinate bonds and covalent bonds, the only real question is how. It is very common in transition metal like platinum, palladium. So, can metals form covalent bonds?
Covalent bonds Learning Lab
Metallic bonds exist in metal crystal lattices. Metals typically do not form covalent bonds. Metals typically have a high number of valence. Having established that there is no real difference between coordinate bonds and covalent bonds, the only real question is how. They usually form ionic bonds with nonmetals.
Online Essay Help amazonia.fiocruz.br
Metals typically have a high number of valence. They usually form ionic bonds with nonmetals. Metallic bonds exist in metal crystal lattices. Having established that there is no real difference between coordinate bonds and covalent bonds, the only real question is how. However, there are exceptions, such as.
How is a covalent bond formed
Metal do form covalent bond. But in other compounds containing a rwo or a few metal atoms, they can be covalently bonded. They usually form ionic bonds with nonmetals. Metals typically do not form covalent bonds. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons.
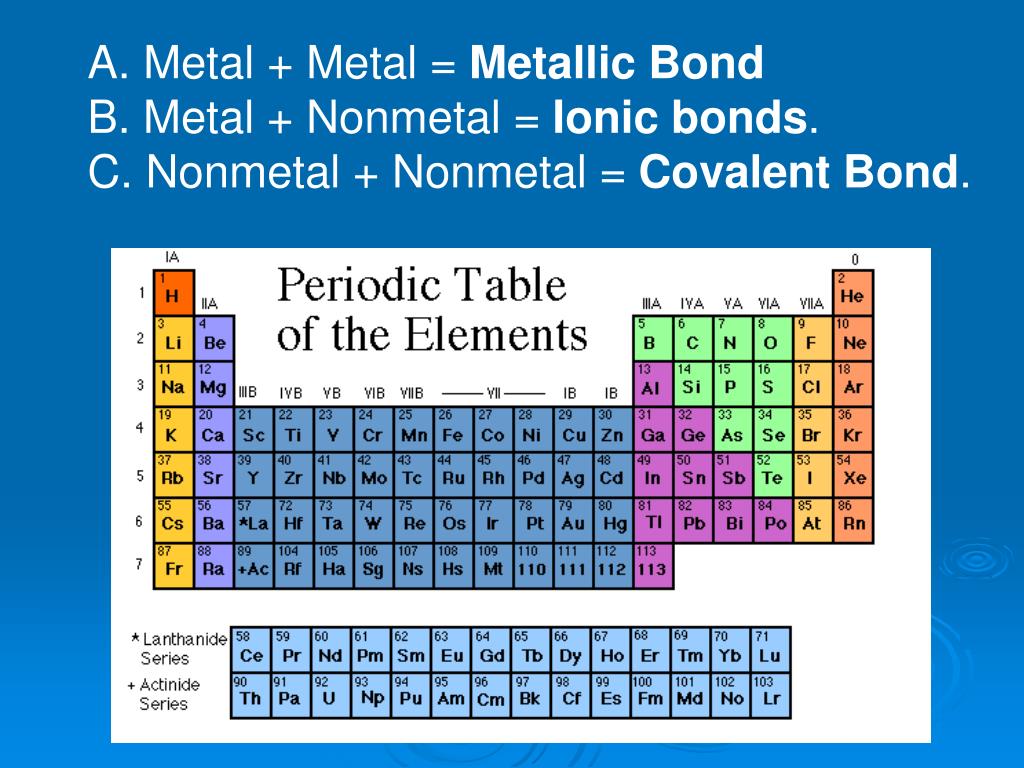
PPT Chapter 1 Chemical Bonding PowerPoint Presentation, free download
Having established that there is no real difference between coordinate bonds and covalent bonds, the only real question is how. However, it is not the way. Metallic bonds exist in metal crystal lattices. They usually form ionic bonds with nonmetals. So, can metals form covalent bonds?
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
It is very common in transition metal like platinum, palladium. The answer is yes, but under specific conditions. However, there are exceptions, such as. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Metals typically do not form covalent bonds.
Metallic Bonding Is A Type Of Chemical Bonding Where Metal Nuclei Share Free Valence Electrons.
However, it is not the way. So, can metals form covalent bonds? They usually form ionic bonds with nonmetals. It is very common in transition metal like platinum, palladium.
The Answer Is Yes, But Under Specific Conditions.
But in other compounds containing a rwo or a few metal atoms, they can be covalently bonded. Metallic bonds exist in metal crystal lattices. Metals typically have a high number of valence. Metal do form covalent bond.
Metals Typically Do Not Form Covalent Bonds.
However, there are exceptions, such as. Having established that there is no real difference between coordinate bonds and covalent bonds, the only real question is how.



:max_bytes(150000):strip_icc()/PeriodicTableElectronegativity-56a12a045f9b58b7d0bca77c.jpg)




:max_bytes(150000):strip_icc()/some-examples-of-covalent-compounds-603981_final21-a3faebbe543e404fb951d2e789031f56.jpg)