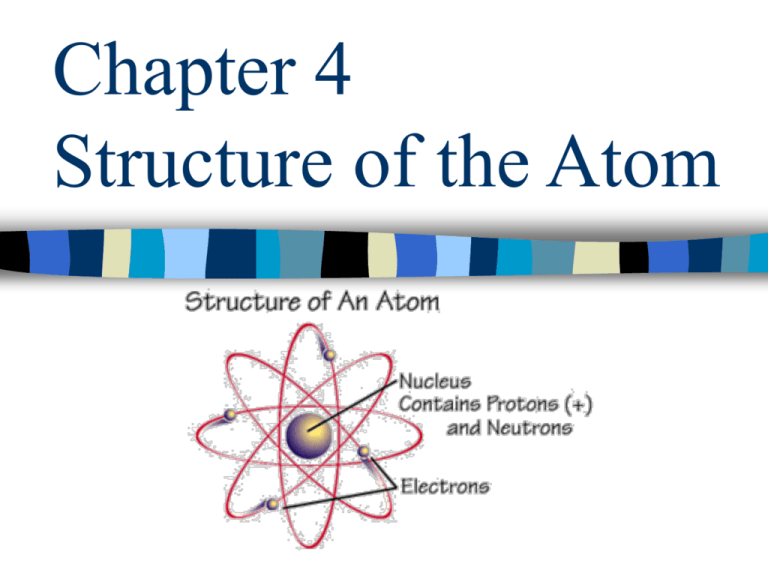
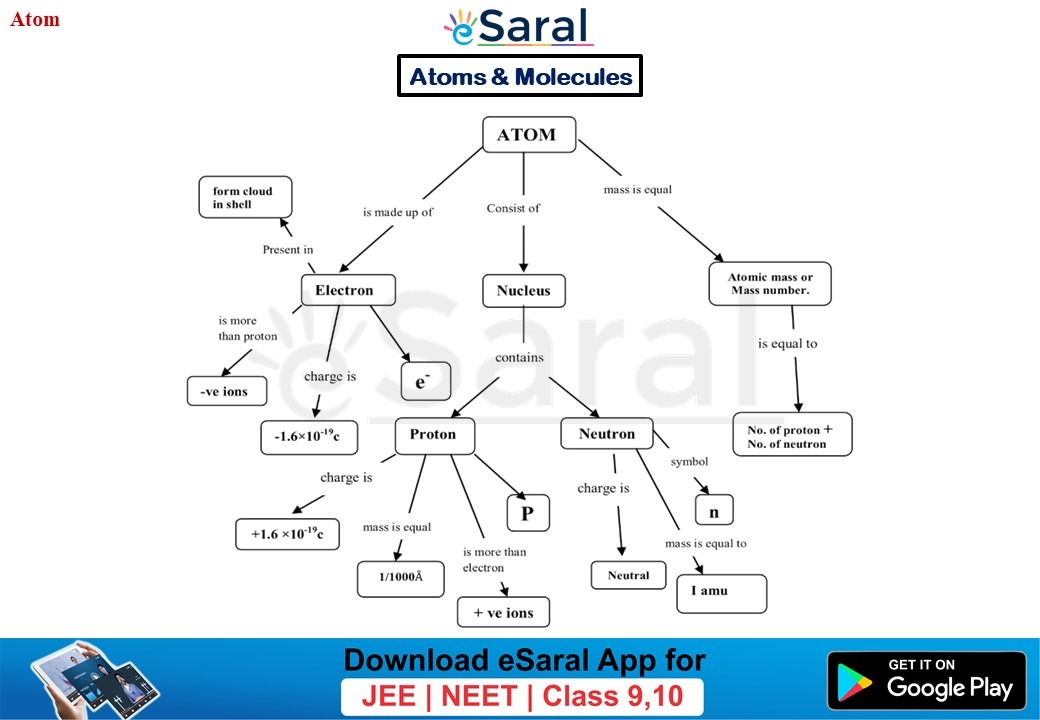
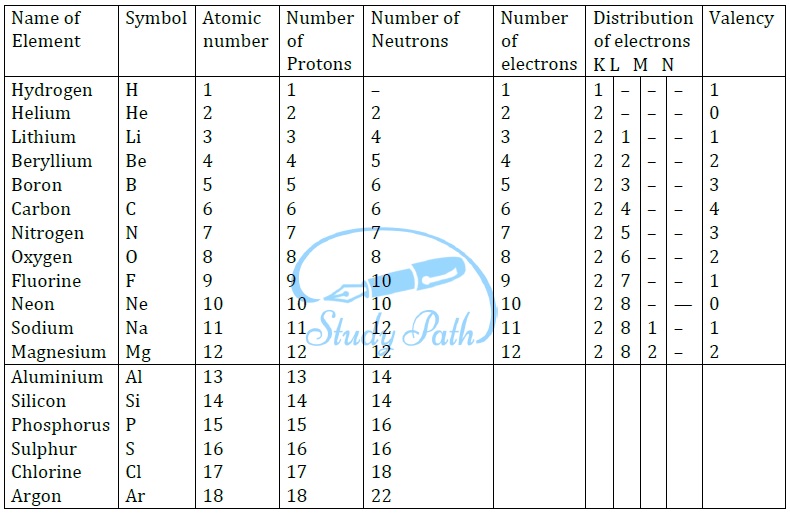
Chapter 4 The Structure Of The Atom - Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons. Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does. Scattering experiments help us study.
Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does. Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons. Scattering experiments help us study.
Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons. Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does. Scattering experiments help us study.
Class 9 Science NCERT Chapter 4 Structure of the Atom YouTube
Scattering experiments help us study. Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons. Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does.
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom
Scattering experiments help us study. Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does. Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons.
NCERT Solutions for Class 9 Science Chapter 4 Structure of Atom
Scattering experiments help us study. Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does. Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons.
C9SC 04A Science Chapter 4 Structure of the Atom Part 1
Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons. Scattering experiments help us study. Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does.
CHAPTER 4 STRUCTURE OF ATOM
Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does. Scattering experiments help us study. Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons.
Chapter 4 Structure of the Atom
Scattering experiments help us study. Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons. Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does.
Chapter 4 Structure of the Atom Pt II YouTube
Scattering experiments help us study. Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons. Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does.
Class Science Chapter Structure Of Atom Study Notes Gurukul, 55 OFF
Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons. Scattering experiments help us study. Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does.
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom
Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does. Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons. Scattering experiments help us study.
Class 9 Science Chapter 4 Structure of Atom Study Notes Gurukul of
Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does. Scattering experiments help us study. Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons.
Rutherford Proposed That An Atom Has A Positively Charged Core (Nucleus) Surrounded By The Negative Electrons.
Scattering experiments help us study. Understanding the structure of the atom is fundamental to understanding why matter behaves the way it does.