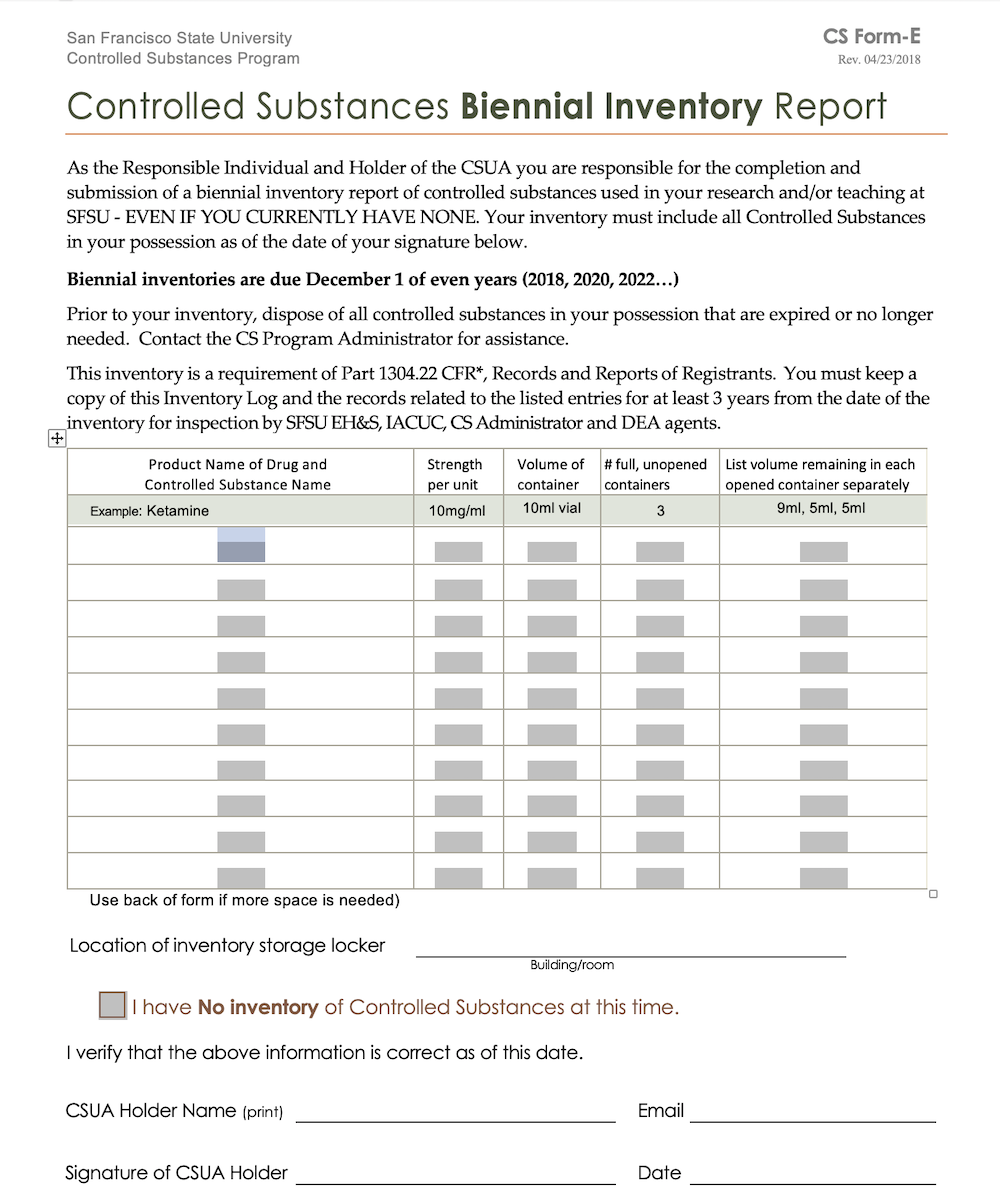
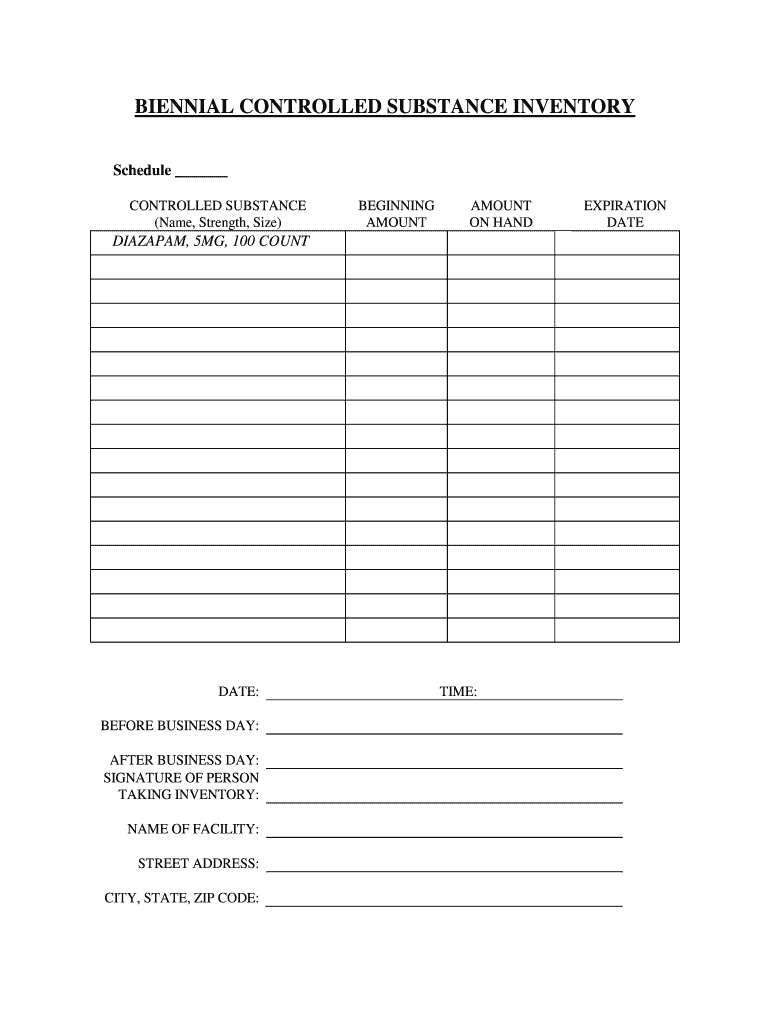
Dea Biennial Inventory Form - (1) cross out the unused lines. The dea requires inventory every two years. This inventory may be taken on any date within two years of the previous date. The dea requires a physical inventory of all controlled substances to be conducted every. Biennial & initial controlled substance inventory form. (2) schedule i and ii drugs. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); (2) schedule i and ii drugs must be separated from all other drugs or. (2) schedule i and ii drugs must be separated from all other drugs or.
This inventory may be taken on any date within two years of the previous date. (1) cross out the unused lines. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires inventory every two years. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); Biennial & initial controlled substance inventory form. (2) schedule i and ii drugs must be separated from all other drugs or. (2) schedule i and ii drugs. The dea requires a physical inventory of all controlled substances to be conducted every.
(1) cross out the unused lines. (2) schedule i and ii drugs must be separated from all other drugs or. (2) schedule i and ii drugs. The dea requires inventory every two years. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. This inventory may be taken on any date within two years of the previous date. The dea requires a physical inventory of all controlled substances to be conducted every. (2) schedule i and ii drugs must be separated from all other drugs or. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); Biennial & initial controlled substance inventory form.
Dea form 41 Fill out & sign online DocHub
(2) schedule i and ii drugs. Biennial & initial controlled substance inventory form. (2) schedule i and ii drugs must be separated from all other drugs or. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); The dea requires a physical inventory of all controlled substances to be conducted every.
Free Printable Controlled Substance Log Printable Word Searches
(2) schedule i and ii drugs. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); (1) cross out the unused lines. The dea requires a physical inventory of all controlled substances to be conducted every. (2) schedule i and ii drugs must be separated from all other drugs or.
CS Form E Biennial Inventory Environment, Health and Safety
The dea requires inventory every two years. Biennial & initial controlled substance inventory form. (1) cross out the unused lines. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. (2) schedule i and ii drugs must be separated from all other drugs or.
Printable Controlled Substance Log 20202022 Fill and Sign Printable
(2) schedule i and ii drugs. Biennial & initial controlled substance inventory form. This inventory may be taken on any date within two years of the previous date. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires inventory every two years.
Use of Controlled Substances in Research Initial Controlled Substance
(2) schedule i and ii drugs must be separated from all other drugs or. (2) schedule i and ii drugs. This inventory may be taken on any date within two years of the previous date. Biennial & initial controlled substance inventory form. The dea requires a physical inventory of all controlled substances to be conducted every.
editable printable controlled substance log fill online printable
Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); The dea requires a physical inventory of all controlled substances to be conducted every. (2) schedule i and ii drugs must be separated from.
Free+Printable+Inventory+Log+Sheet
(2) schedule i and ii drugs must be separated from all other drugs or. (2) schedule i and ii drugs. (1) cross out the unused lines. (2) schedule i and ii drugs must be separated from all other drugs or. This inventory may be taken on any date within two years of the previous date.
Dea biennial inventory form Fill out & sign online DocHub
This inventory may be taken on any date within two years of the previous date. The dea requires a physical inventory of all controlled substances to be conducted every. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial.
Dea biennial inventory form Fill out & sign online DocHub
(2) schedule i and ii drugs must be separated from all other drugs or. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); This inventory may be taken on any date within two years of the previous date. (2) schedule i and ii drugs must be separated from all other drugs or. Dea biennial controlled.
SOLUTION Cs biennial inventory form Studypool
(1) cross out the unused lines. (2) schedule i and ii drugs must be separated from all other drugs or. Biennial & initial controlled substance inventory form. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate.
(2) Schedule I And Ii Drugs Must Be Separated From All Other Drugs Or.
(2) schedule i and ii drugs. Biennial & initial controlled substance inventory form. This inventory may be taken on any date within two years of the previous date. (1) cross out the unused lines.
Dea Biennial Controlled Substance Inventory Form For The Use Of Controlled Substances In Research A Separate Initial Inventory Is Required For Each.
The dea requires a physical inventory of all controlled substances to be conducted every. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires inventory every two years. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients);