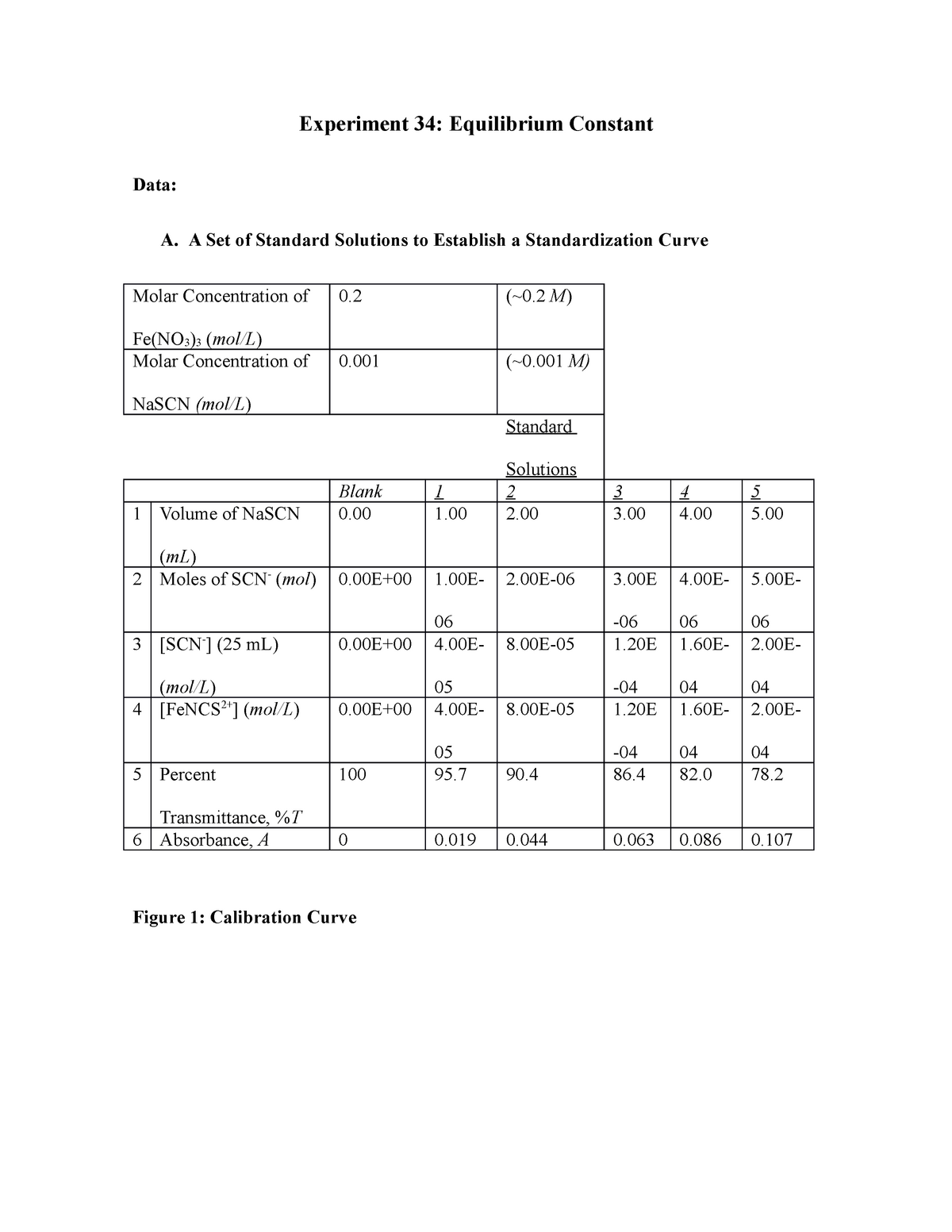
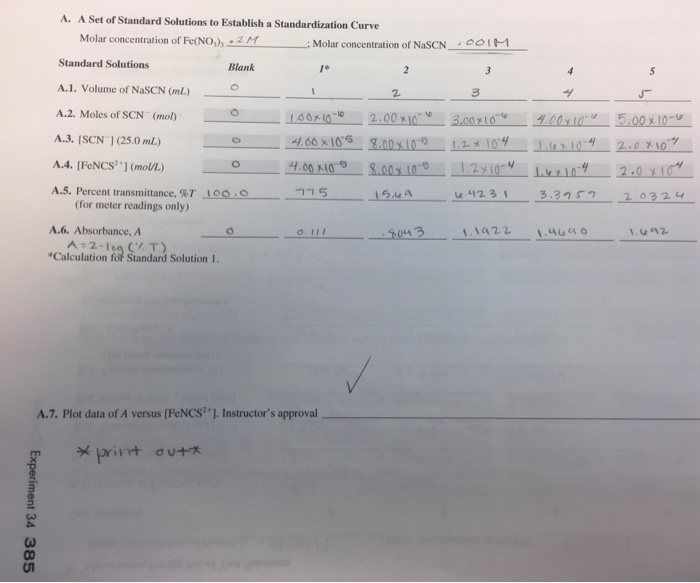
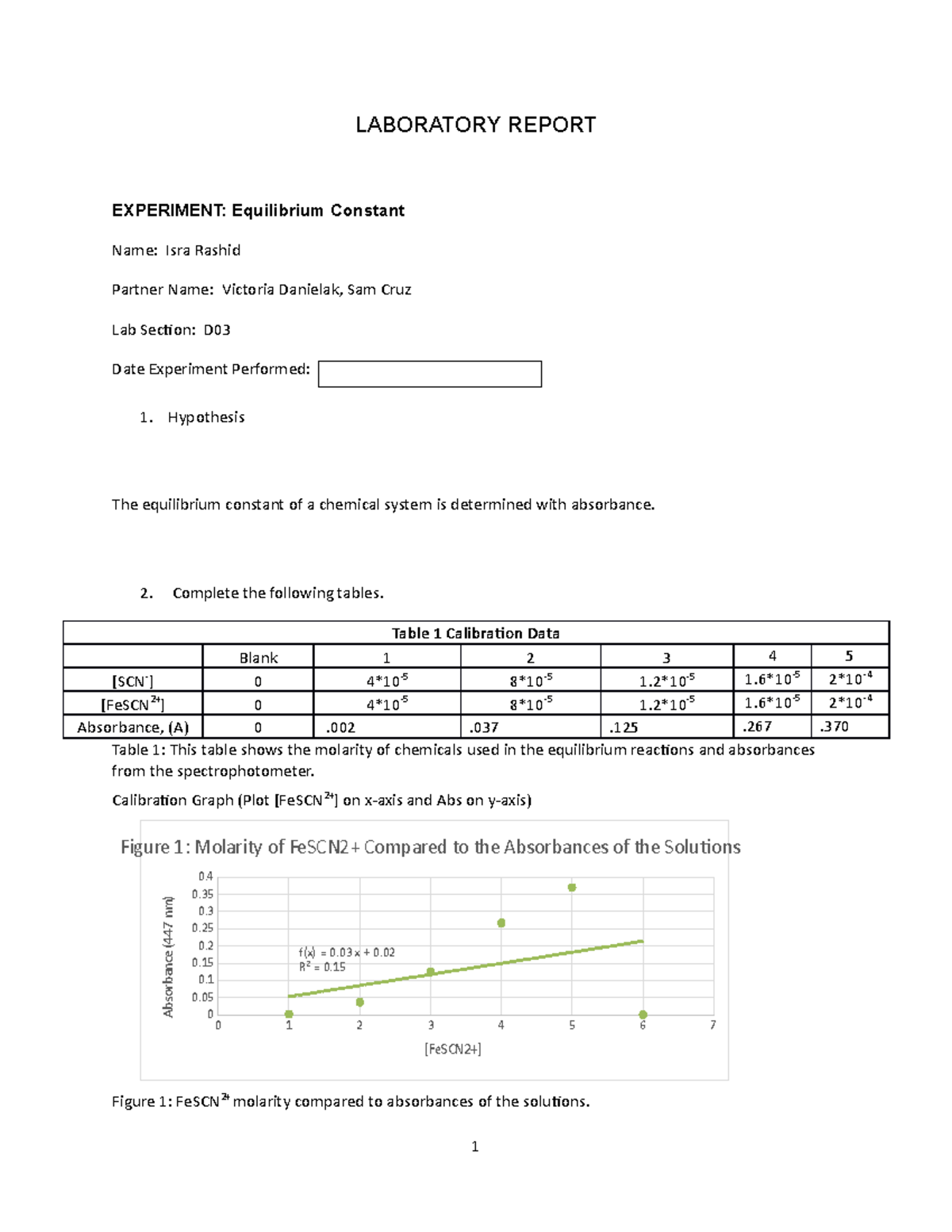
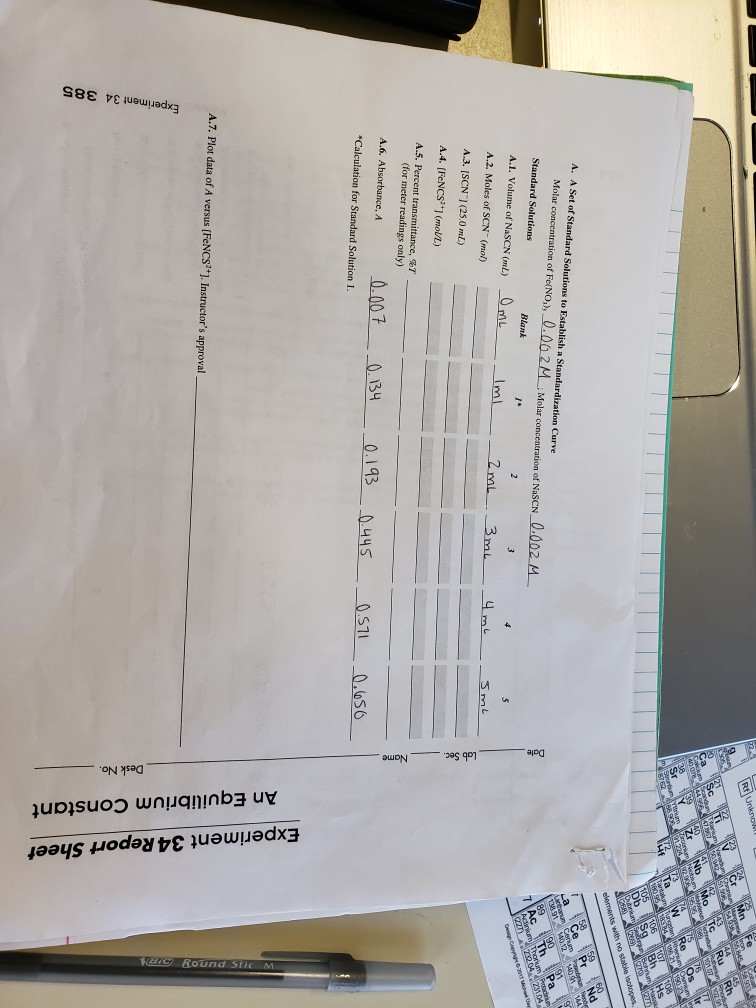
Experiment 34 An Equilibrium Constant Report Sheet - A numerical value representing the ratio of products to reactants at equilibrium. Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Experiment 34, detailed in this report,. What is an equilibrium constant?
Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. A numerical value representing the ratio of products to reactants at equilibrium. What is an equilibrium constant? Experiment 34, detailed in this report,. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing.
Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Experiment 34, detailed in this report,. A numerical value representing the ratio of products to reactants at equilibrium. What is an equilibrium constant?
Solved Experiment 34 ratory Assignment An Equilibrium
A numerical value representing the ratio of products to reactants at equilibrium. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. Experiment 34, detailed in this report,. What is an equilibrium constant?
Solved The next questions are related to Experiment 34.
Experiment 34, detailed in this report,. A numerical value representing the ratio of products to reactants at equilibrium. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. What is an equilibrium constant? Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants.
LAB Report; Labster Equilibrium GENERAL CHEMISTRY 2 EXPERIMENT NO. 5
Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Experiment 34, detailed in this report,. What is an equilibrium constant? Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. A numerical value representing the ratio of products to reactants at equilibrium.
Experiment 34 An Equilibrium Constant YouTube
Experiment 34, detailed in this report,. What is an equilibrium constant? Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. A numerical value representing the ratio of products to reactants at equilibrium. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing.
Equilibrium Constant Lab Answers
What is an equilibrium constant? Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. A numerical value representing the ratio of products to reactants at equilibrium. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Experiment 34, detailed in this report,.
Solved Please help me with part C. I posted part A, B and
Experiment 34, detailed in this report,. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. A numerical value representing the ratio of products to reactants at equilibrium. What is an equilibrium constant? Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants.
Solved Experiment 34 Prelaboratory Assignment An Equilibrium
Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. A numerical value representing the ratio of products to reactants at equilibrium. Experiment 34, detailed in this report,. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. What is an equilibrium constant?
Exp 34 Equilibrium Constant numbers only LABORATORY REPORT EXPERIMENT
A numerical value representing the ratio of products to reactants at equilibrium. Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. Experiment 34, detailed in this report,. What is an equilibrium constant? Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing.
Experiment 34 Report Sheet An Equilibrium Constant
Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. A numerical value representing the ratio of products to reactants at equilibrium. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Experiment 34, detailed in this report,. What is an equilibrium constant?
Experiment 34 An Equilibrium Constant
Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. What is an equilibrium constant? A numerical value representing the ratio of products to reactants at equilibrium. Experiment 34, detailed in this report,. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing.
Equilibrium Favors The Products, While A Small K Value Indicates That The Equilibrium Favors The Reactants.
Experiment 34, detailed in this report,. What is an equilibrium constant? A numerical value representing the ratio of products to reactants at equilibrium. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing.