Fda Form 3911 - Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. The form includes information on product. Refer to instruction sheet (form fda 3911 supplement) for more information. Form fda 3911 is used for drug notification purposes. Type of report (select one): The guidance also addresses how trading partners should notify fda using form fda 3911.
Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. The form includes information on product. The guidance also addresses how trading partners should notify fda using form fda 3911. Form fda 3911 is used for drug notification purposes. Type of report (select one): Refer to instruction sheet (form fda 3911 supplement) for more information.
Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. Type of report (select one): The guidance also addresses how trading partners should notify fda using form fda 3911. Refer to instruction sheet (form fda 3911 supplement) for more information. Form fda 3911 is used for drug notification purposes. The form includes information on product.
Printable 3911 Form Printable Templates
Type of report (select one): Form fda 3911 is used for drug notification purposes. The form includes information on product. Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. Refer to instruction sheet (form fda 3911 supplement) for more information.
FORM FDA 3911 Instructional Supplement Instructions for Completion of
Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. Form fda 3911 is used for drug notification purposes. Refer to instruction sheet (form fda 3911 supplement) for more information. Type of report (select one): The guidance also addresses how trading partners should notify fda using form fda.
K190909 FDA Form 3881 Medical Device Academy
The form includes information on product. Refer to instruction sheet (form fda 3911 supplement) for more information. Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. Type of report (select one): The guidance also addresses how trading partners should notify fda using form fda 3911.
Irs Form 3911 Printable prntbl.concejomunicipaldechinu.gov.co
Form fda 3911 is used for drug notification purposes. Refer to instruction sheet (form fda 3911 supplement) for more information. The form includes information on product. Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. The guidance also addresses how trading partners should notify fda using form.
Steps to Consider When Dealing With 483 FDA and Warning Letters Dot
The guidance also addresses how trading partners should notify fda using form fda 3911. Form fda 3911 is used for drug notification purposes. The form includes information on product. Type of report (select one): Refer to instruction sheet (form fda 3911 supplement) for more information.
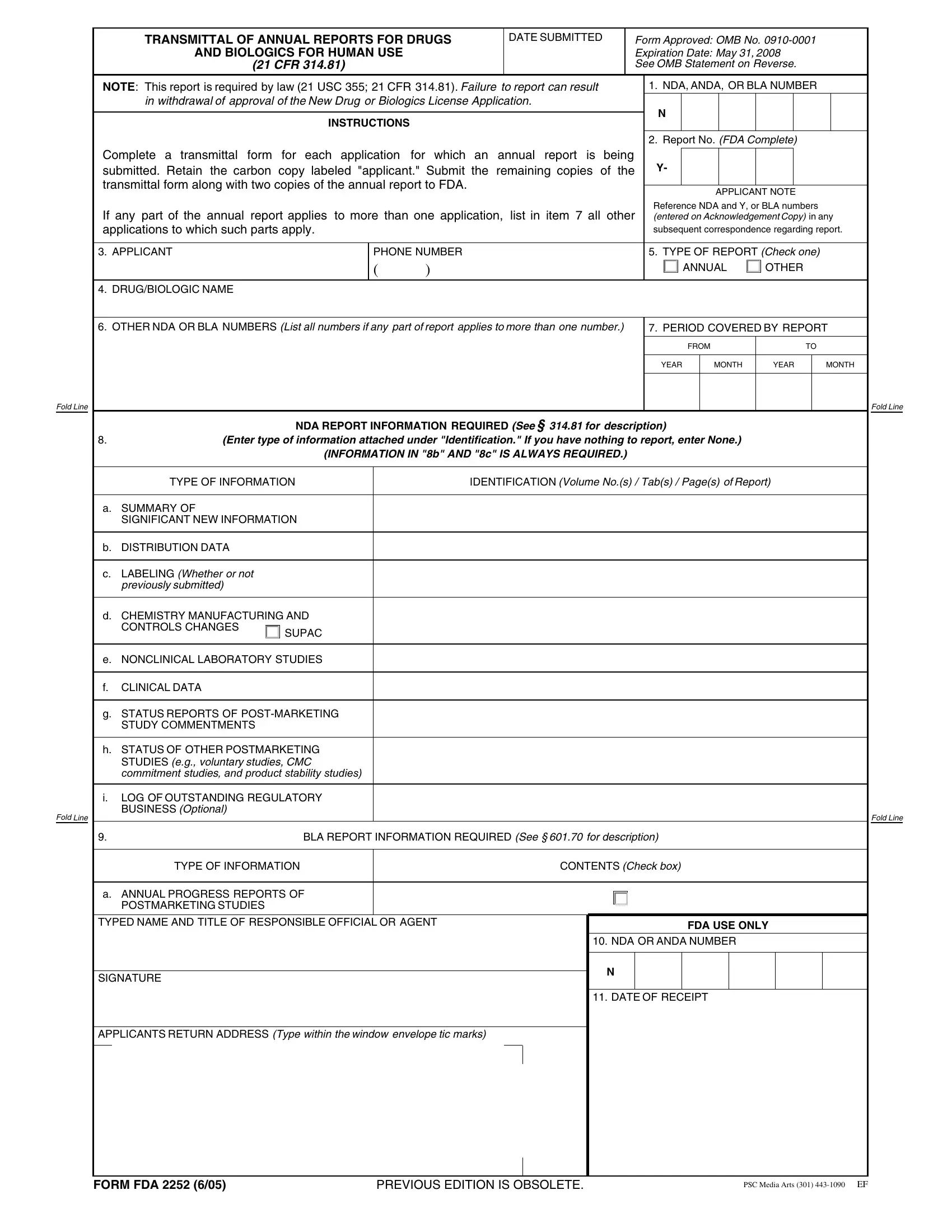
Form Fda 2252 ≡ Fill Out Printable PDF Forms Online
Refer to instruction sheet (form fda 3911 supplement) for more information. Form fda 3911 is used for drug notification purposes. Type of report (select one): Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. The guidance also addresses how trading partners should notify fda using form fda.
Form 3500 Fda Complete with ease airSlate SignNow
Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. The guidance also addresses how trading partners should notify fda using form fda 3911. Refer to instruction sheet (form fda 3911 supplement) for more information. The form includes information on product. Form fda 3911 is used for drug.
Form 3911 Printable
Refer to instruction sheet (form fda 3911 supplement) for more information. The form includes information on product. Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. Type of report (select one): Form fda 3911 is used for drug notification purposes.
Form Fda 3419 ≡ Fill Out Printable PDF Forms Online
Form fda 3911 is used for drug notification purposes. The form includes information on product. Refer to instruction sheet (form fda 3911 supplement) for more information. Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. Type of report (select one):
Fillable Online FORM FDA 3911. Drug Notification Fax Email Print
Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. The guidance also addresses how trading partners should notify fda using form fda 3911. Form fda 3911 is used for drug notification purposes. Type of report (select one): The form includes information on product.
Form Fda 3911 Is Used For Drug Notification Purposes.
Fda is announcing the expansion of the fda cder nextgen portal to include a 3911 platform that enables trading partners to submit. Refer to instruction sheet (form fda 3911 supplement) for more information. The guidance also addresses how trading partners should notify fda using form fda 3911. Type of report (select one):