Gamp 5 Full Form - Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Good automated manufacturing practice, founded in 1991. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. What are gamp® 5 categories, requirements, and.
Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Good automated manufacturing practice, founded in 1991. What are gamp® 5 categories, requirements, and. Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the.
Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. What are gamp® 5 categories, requirements, and. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Good automated manufacturing practice, founded in 1991.
GAMP 5 and GAMP 5 2nd Edition What are the main differences? Scilife
Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Good automated manufacturing practice, founded in 1991..
GAMP5 GUIDELINES PDF
Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. Good automated manufacturing practice, founded in 1991. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing.
GAMP 5 and GAMP 5 2nd Edition What are the main differences? Scilife
What are gamp® 5 categories, requirements, and. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Good automated manufacturing practice, founded in 1991. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Gamp 5 provides a structured approach to the validation of computerized systems.
GAMP 5 Uncovered What You Need to Know Scilife
The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Good automated manufacturing practice, founded in 1991. What are gamp® 5 categories, requirements, and. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Gamp 5 provides a structured approach to the validation of computerized systems.
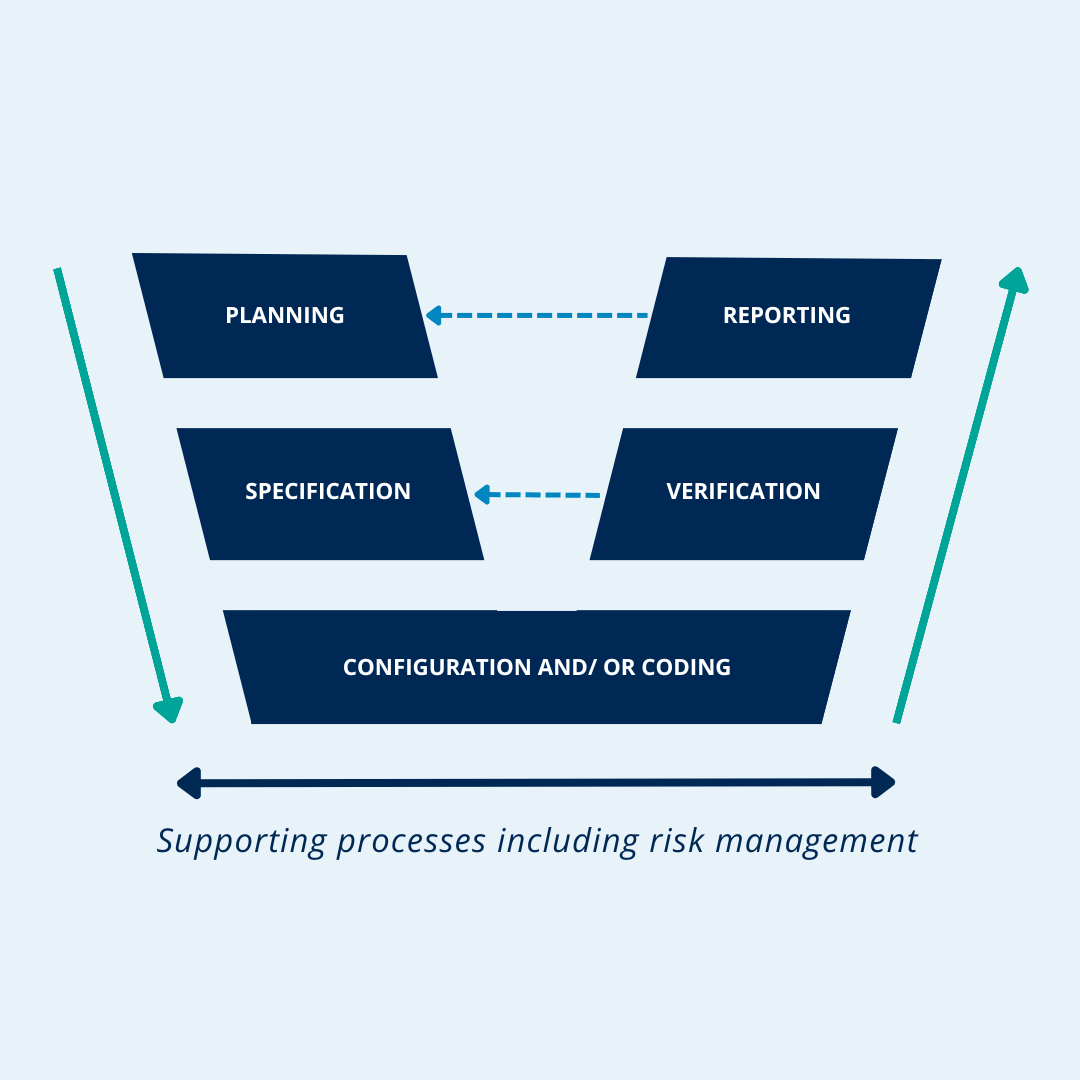
What is the GAMP 5 Vmodel in CSV? QbD Group
Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Good automated manufacturing practice, founded in 1991..
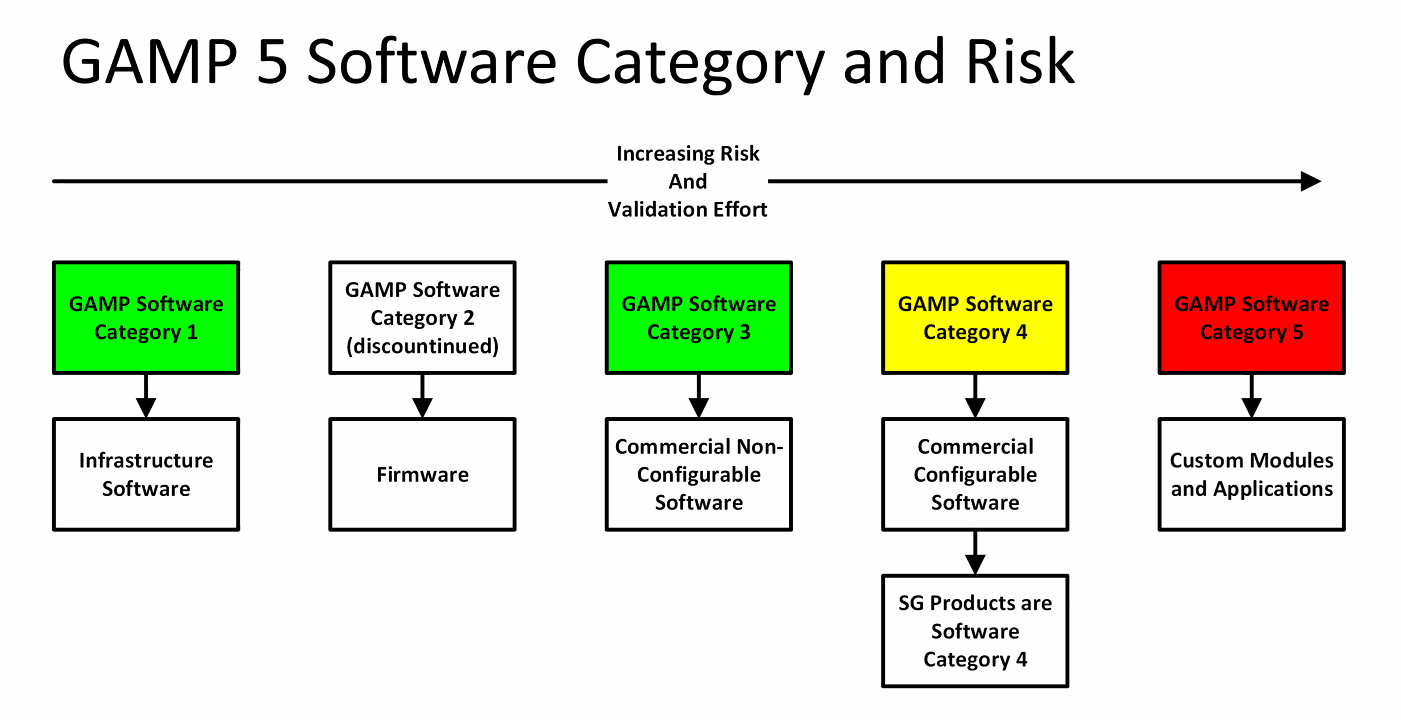
V5 GAMP 5 Software Category & Risk SG Systems Global
Good automated manufacturing practice, founded in 1991. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. What are gamp® 5 categories, requirements, and. Gamp 5 provides a structured.
GAMP 5 Uncovered What You Need to Know Scilife
Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Good automated manufacturing practice, founded in 1991. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems..
GAMP 5 and GAMP 5 2nd Edition What are the main differences? Scilife
The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. What are gamp® 5 categories, requirements, and. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of.
What is the GAMP 5 Vmodel in CSV? QbD Group
What are gamp® 5 categories, requirements, and. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Good automated manufacturing practice, founded in 1991. Gamp 5 provides a structured approach to the validation of computerized systems used.
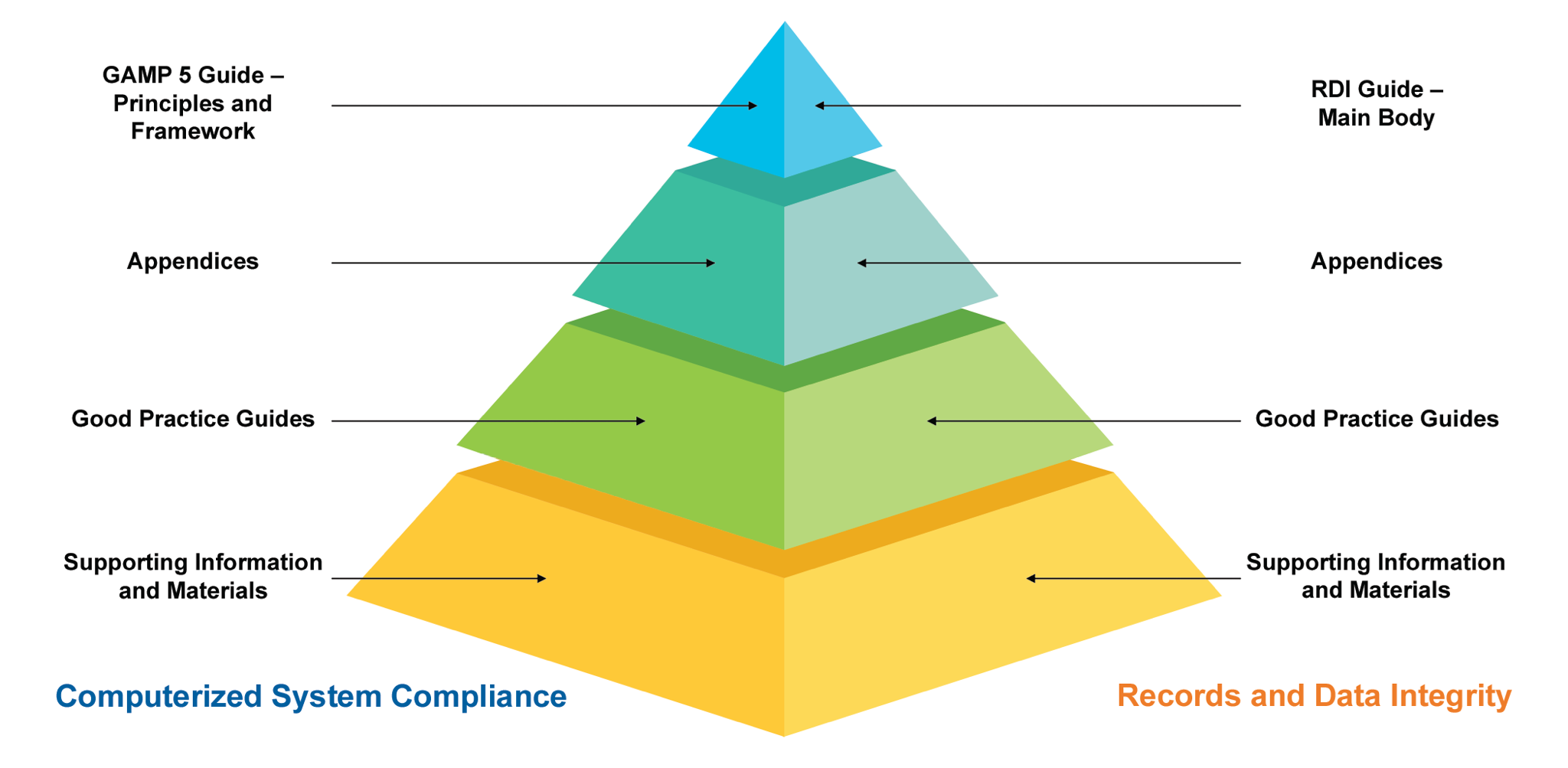
ISPE GAMP5 second edition What’s new?
Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Good automated manufacturing practice, founded in 1991. Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems..
What Are Gamp® 5 Categories, Requirements, And.
The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Good automated manufacturing practice, founded in 1991.