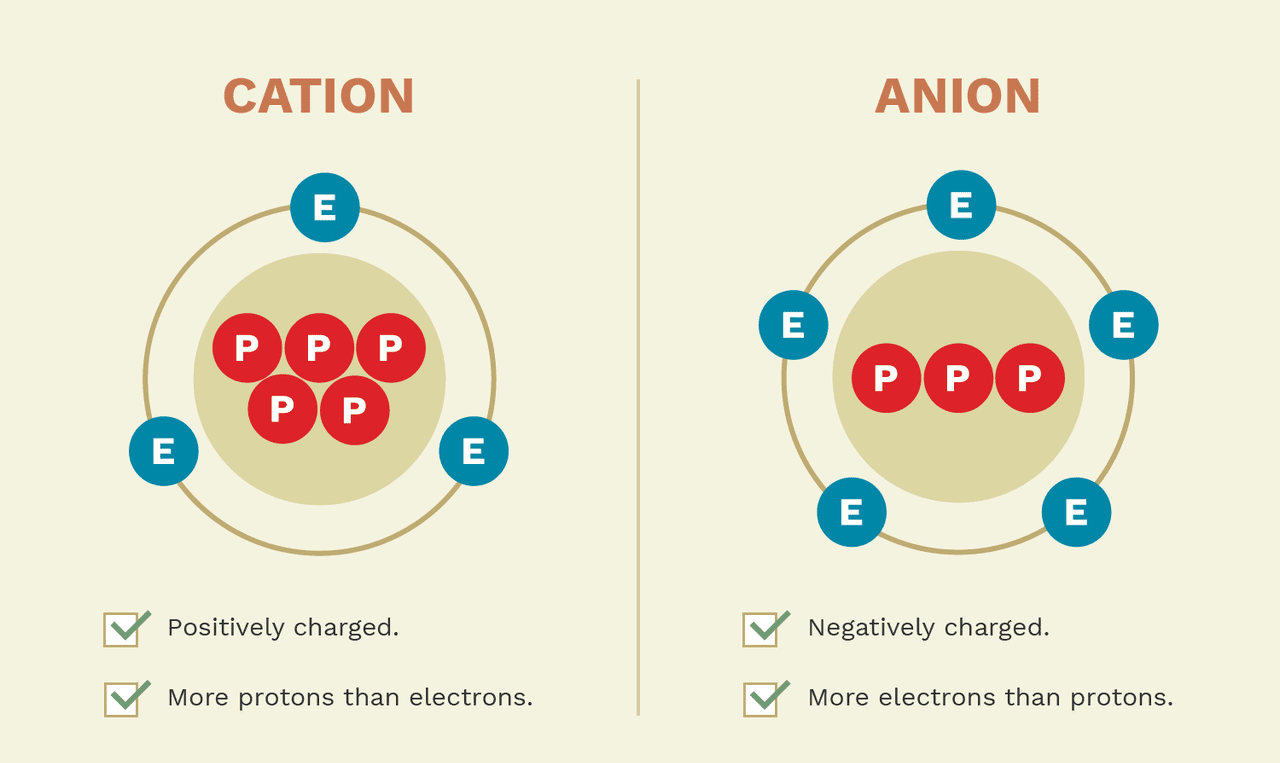
How Cations Are Formed - Negatively charged ions formed as a result of gaining. A cation is formed from an atom that loses one or more electrons. Cations and anions form ionic bonds because of the. Positively charged ions formed as a result of losing electrons. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for.
Positively charged ions formed as a result of losing electrons. Negatively charged ions formed as a result of gaining. A cation is formed from an atom that loses one or more electrons. Cations and anions form ionic bonds because of the. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for.
Negatively charged ions formed as a result of gaining. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for. A cation is formed from an atom that loses one or more electrons. Positively charged ions formed as a result of losing electrons. Cations and anions form ionic bonds because of the.
Amazing Tricks To Understanding Cations and Anions Key Concepts and
Cations and anions form ionic bonds because of the. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for. A cation is formed from an atom that loses one or more electrons. Positively charged ions formed as a result of losing electrons. Negatively charged ions formed.
Difference Between Cation and Anion
Positively charged ions formed as a result of losing electrons. Negatively charged ions formed as a result of gaining. A cation is formed from an atom that loses one or more electrons. Cations and anions form ionic bonds because of the. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of.
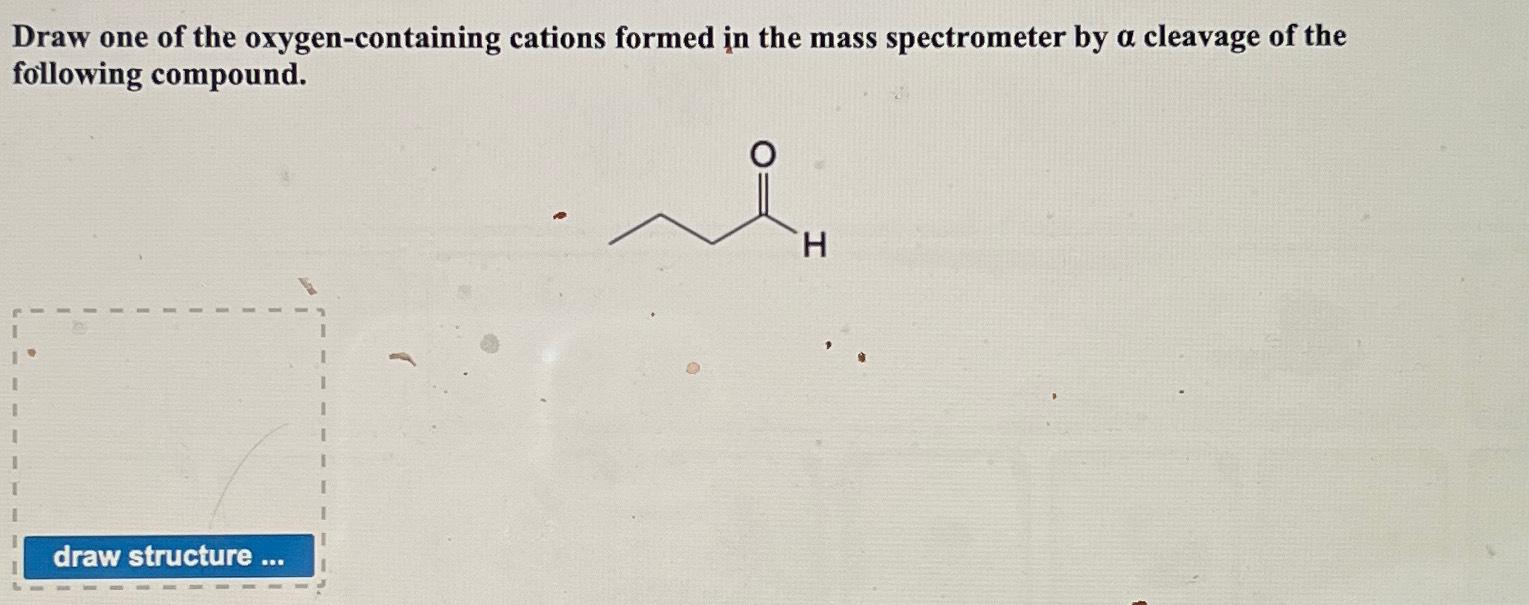
Solved Draw one of the oxygencontaining cations formed in
Cations and anions form ionic bonds because of the. Negatively charged ions formed as a result of gaining. Positively charged ions formed as a result of losing electrons. A cation is formed from an atom that loses one or more electrons. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of.
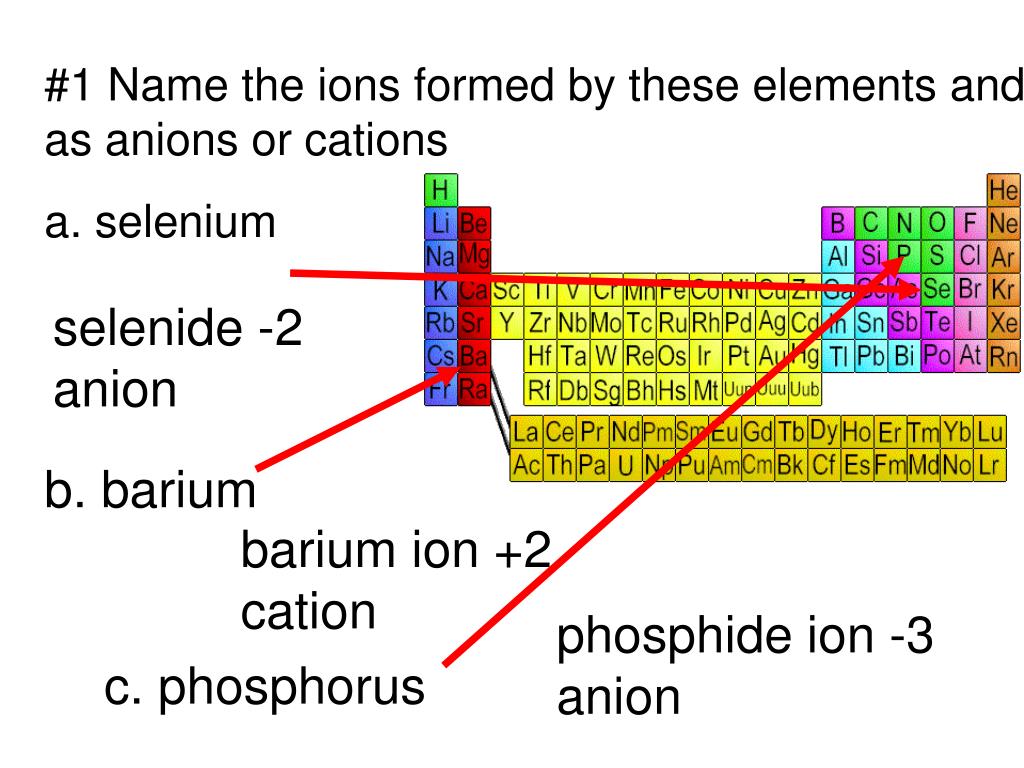
PPT 1 Name the ions formed by these elements and classify them as
Negatively charged ions formed as a result of gaining. A cation is formed from an atom that loses one or more electrons. Positively charged ions formed as a result of losing electrons. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for. Cations and anions form.
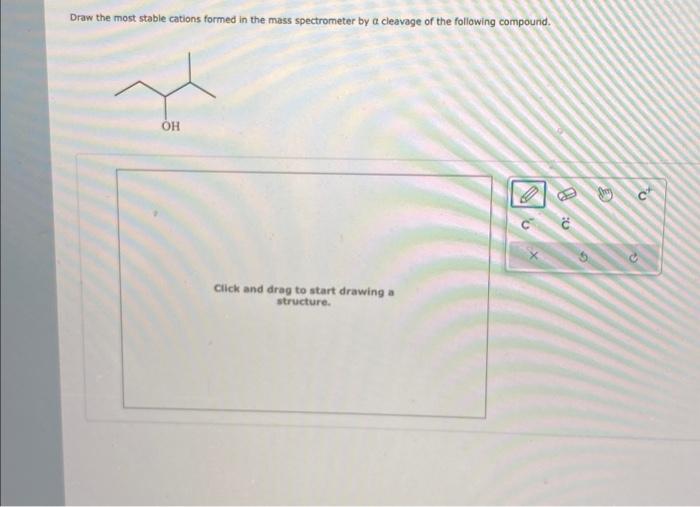
Solved Draw the most stable cations formed in the mass
Cations and anions form ionic bonds because of the. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for. A cation is formed from an atom that loses one or more electrons. Positively charged ions formed as a result of losing electrons. Negatively charged ions formed.
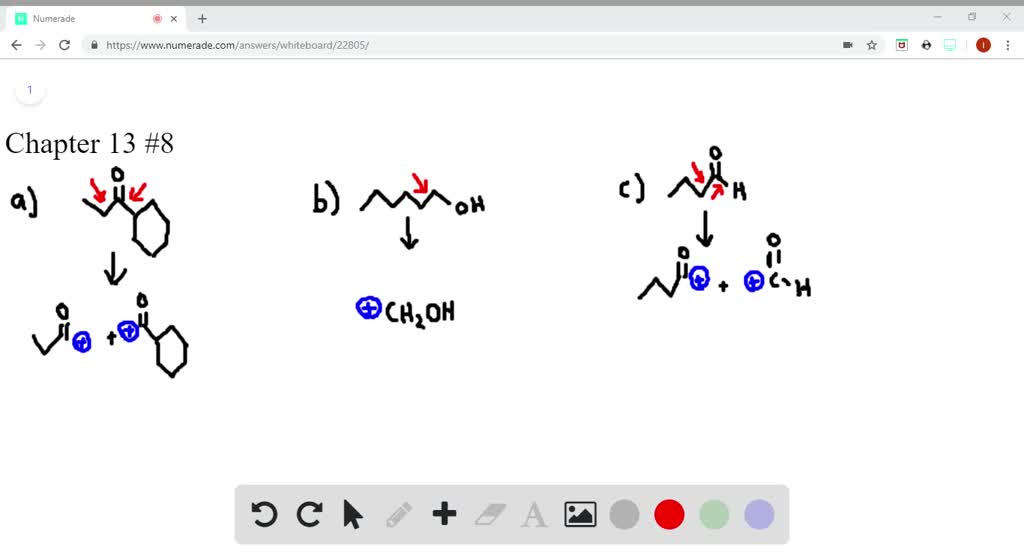
SOLVEDWhat cations are formed in the mass spectrometer by αcleavage of
A cation is formed from an atom that loses one or more electrons. Negatively charged ions formed as a result of gaining. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for. Cations and anions form ionic bonds because of the. Positively charged ions formed as.
Solved Draw the most stable cations formed in the mass
A cation is formed from an atom that loses one or more electrons. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for. Positively charged ions formed as a result of losing electrons. Negatively charged ions formed as a result of gaining. Cations and anions form.
Cations and Anions Definitions, Examples, and Differences
A cation is formed from an atom that loses one or more electrons. Positively charged ions formed as a result of losing electrons. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for. Cations and anions form ionic bonds because of the. Negatively charged ions formed.
Diferencia entre aniónes y catiónes (con nomenclatura y ejemplos
Positively charged ions formed as a result of losing electrons. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for. Negatively charged ions formed as a result of gaining. Cations and anions form ionic bonds because of the. A cation is formed from an atom that.
Cations and Anions Difference between Cations and Anions
Cations and anions form ionic bonds because of the. Positively charged ions formed as a result of losing electrons. A cation is formed from an atom that loses one or more electrons. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for. Negatively charged ions formed.
A Cation Is Formed From An Atom That Loses One Or More Electrons.
Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for. Positively charged ions formed as a result of losing electrons. Negatively charged ions formed as a result of gaining. Cations and anions form ionic bonds because of the.