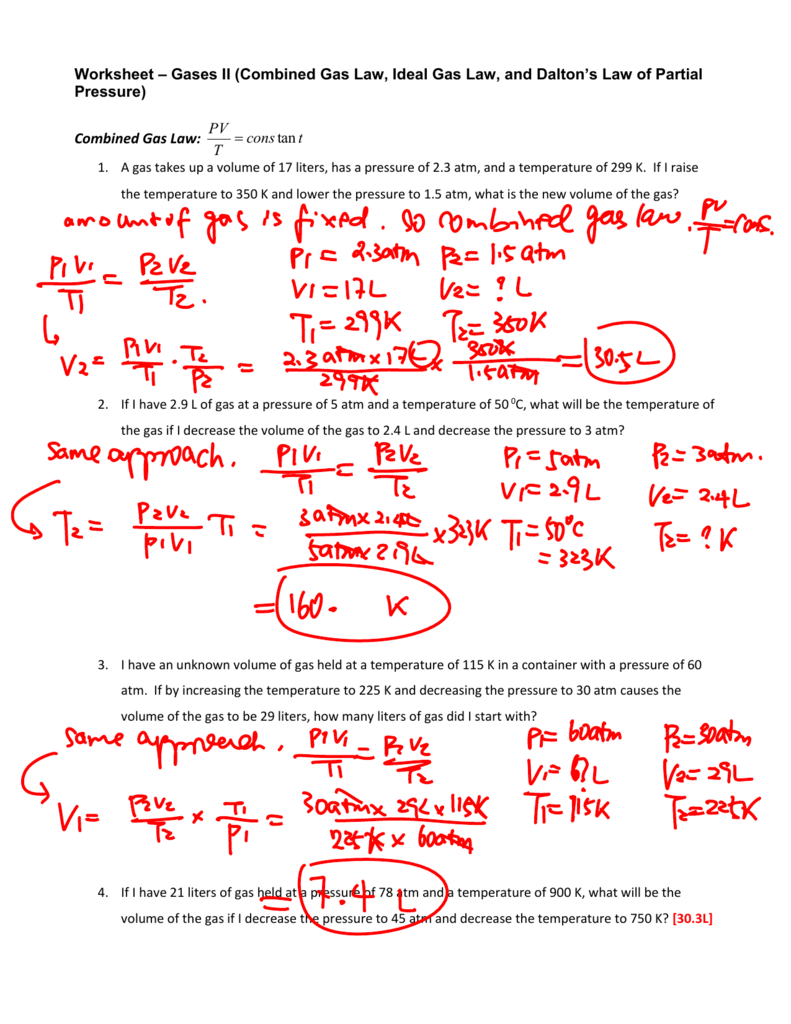
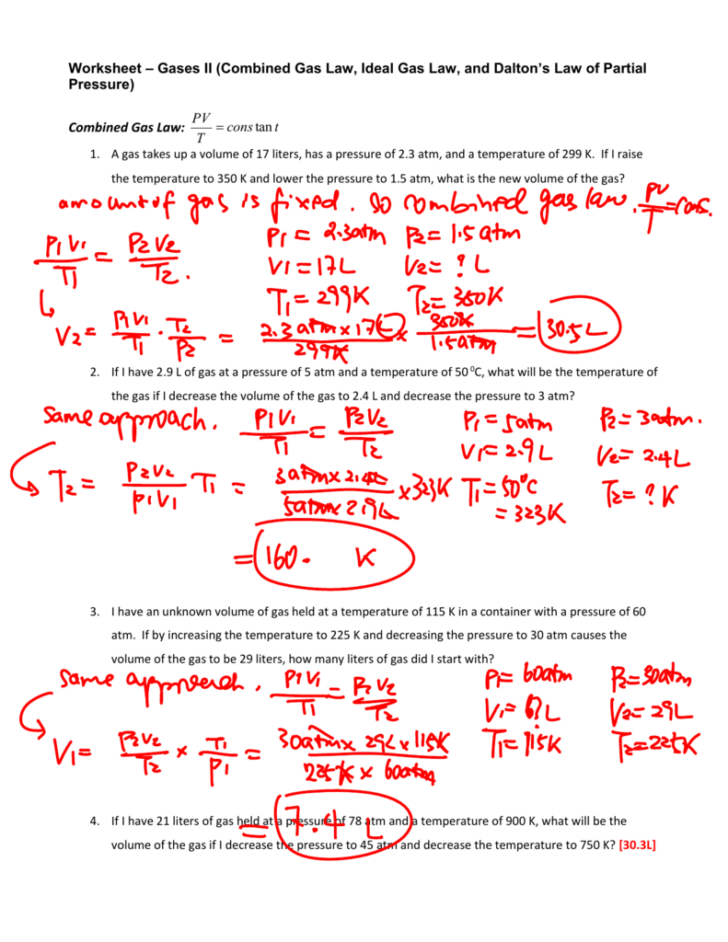
Ideal Gas Law Worksheet With Answers - Show your work, including proper units, to earn full credit. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. Solve each of the following problems. On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships between the. Use your knowledge of the ideal and combined gas laws to solve the following problems. If it involves moles or grams, it must be pv = nrt. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? 10 ideal gas law 1. The ideal gas law directions:
Use your knowledge of the ideal and combined gas laws to solve the following problems. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships between the. Show your work, including proper units, to earn full credit. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. The ideal gas law directions: Solve each of the following problems. If it involves moles or grams, it must be pv = nrt. 10 ideal gas law 1.
If it involves moles or grams, it must be pv = nrt. Solve each of the following problems. 10 ideal gas law 1. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. Show your work, including proper units, to earn full credit. The ideal gas law directions: How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships between the. Use your knowledge of the ideal and combined gas laws to solve the following problems.
Worksheet Gas Laws II Answers
The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. The ideal gas law directions: On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships between.
30++ Combined Gas Law Worksheet Answer Key Worksheets Decoomo
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Show your work, including proper units, to earn full credit. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas.
4 Ideal Gas Law Worksheet FabTemplatez
On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships between the. The ideal gas law directions: Show your work, including proper units, to earn full credit. Use your knowledge of the ideal and combined gas laws to solve the following problems. Solve each of the following problems.
20++ Ideal Gas Law Worksheet Answers Worksheets Decoomo
Use your knowledge of the ideal and combined gas laws to solve the following problems. Solve each of the following problems. 10 ideal gas law 1. If it involves moles or grams, it must be pv = nrt. On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships between the.
Combined Gas Law Worksheet Answers Printable Word Searches
10 ideal gas law 1. Solve each of the following problems. Use your knowledge of the ideal and combined gas laws to solve the following problems. Show your work, including proper units, to earn full credit. The ideal gas law directions:
20++ Ideal Gas Law Worksheet Answers Worksheets Decoomo
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The ideal gas law directions: Use your knowledge of the ideal and combined gas laws to solve the following problems. On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships.
Ideal Gas Law Worksheet 2 Answer Ideal Gas Law Worksheet PV = nRT Use
10 ideal gas law 1. If it involves moles or grams, it must be pv = nrt. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Use your knowledge of the ideal and combined gas laws to solve the following problems. On this worksheet you will practice with the.
Ideal Gas Law Worksheet Answer Key —
Solve each of the following problems. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. 10 ideal gas law 1. Show your work, including proper units, to earn full credit. Use your knowledge of the.
Solved Ideal Gas Law Practice Worksheet Solve the following
Solve each of the following problems. Show your work, including proper units, to earn full credit. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. 10 ideal gas law 1. How many moles of gas.
30++ Gas Laws Worksheet 1 Answer Key Worksheets Decoomo
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? If it involves moles or grams, it must be pv = nrt. Solve each of the following problems. Use your knowledge of the ideal and combined gas laws to solve the following problems. Show your work, including proper units, to.
Solve Each Of The Following Problems.
If it involves moles or grams, it must be pv = nrt. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Show your work, including proper units, to earn full credit. Use your knowledge of the ideal and combined gas laws to solve the following problems.
On This Worksheet You Will Practice With The Ideal Gas Law, The Combined Gas Law, As Well As The Relationships Between The.
10 ideal gas law 1. The ideal gas law directions: The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the.