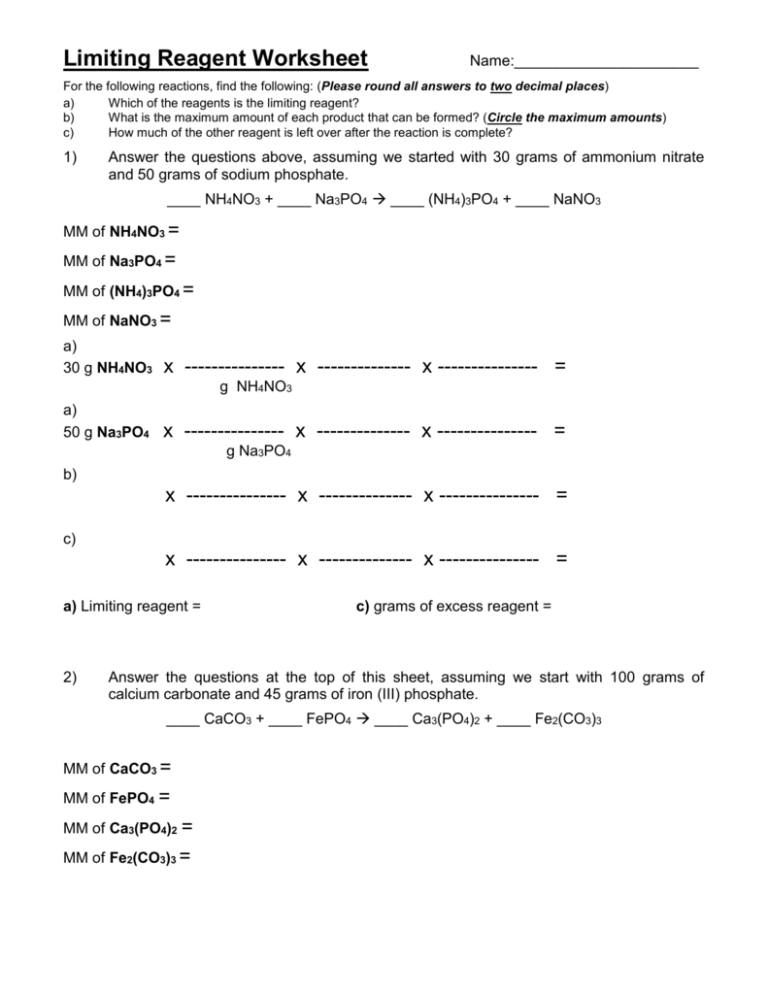
Limiting Reactant Worksheet 1 - How many moles of nh3 can be produced from the reaction of 28 g of n2 ? (hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if you start with 14.8 g of c 3h 8. (a) identify the limiting reactant. A student places 2.36 moles of acetic acid. Worksheet on excess and limiting reactants/reagents. If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. Include work and units for full credit 1. What is a limiting reactant? Determine the number of moles of al produced. (b) how many moles of carbon dioxide gas (co2) will form?
(hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if you start with 14.8 g of c 3h 8. What is a limiting reactant? (b) how many moles of carbon dioxide gas (co2) will form? Worksheet on excess and limiting reactants/reagents. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Determine the number of moles of al produced. A student places 2.36 moles of acetic acid. (a) identify the limiting reactant. Include work and units for full credit 1. When dealing with questions where the mol of more than one reactant is.
(b) how many moles of carbon dioxide gas (co2) will form? Determine the number of moles of al produced. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Worksheet on excess and limiting reactants/reagents. When dealing with questions where the mol of more than one reactant is. A student places 2.36 moles of acetic acid. Include work and units for full credit 1. What is a limiting reactant? If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. (a) identify the limiting reactant.
Limiting Reactants Worksheet
A student places 2.36 moles of acetic acid. Include work and units for full credit 1. (b) how many moles of carbon dioxide gas (co2) will form? If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. Determine the number of moles of al produced.
Limiting Reagent Worksheet 1 Answers trendstoday
What is a limiting reactant? (hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if you start with 14.8 g of c 3h 8. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Determine the number of moles of al produced. (b) how many moles.
Limiting Reagent Worksheet 1 Answers trendstoday
Worksheet on excess and limiting reactants/reagents. If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. What is a limiting reactant? (a) identify the limiting reactant. A student places 2.36 moles of acetic acid.
Limiting Reagent Worksheet 1
(hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if you start with 14.8 g of c 3h 8. Include work and units for full credit 1. Worksheet on excess and limiting reactants/reagents. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Determine the number.
Limiting And Excess Reactants Worksheet Key
(hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if you start with 14.8 g of c 3h 8. Worksheet on excess and limiting reactants/reagents. A student places 2.36 moles of acetic acid. What is a limiting reactant? If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting.
Limiting Reactant Worksheet 1 Printable Word Searches
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? A student places 2.36 moles of acetic acid. What is a limiting reactant? Include work and units for full credit 1. (hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if you start with 14.8 g.
Limiting Reactant Worksheet
Include work and units for full credit 1. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. (hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if you start.
Limiting Reagents Worksheets
What is a limiting reactant? If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. (a) identify the limiting reactant. When dealing with questions where the mol of more than one reactant is. How many moles of nh3 can be produced from the reaction of 28 g of n2 ?
Limiting Reagent Worksheet 1
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? (b) how many moles of carbon dioxide gas (co2) will form? Determine the number of moles of al produced. What is a limiting reactant? Include work and units for full credit 1.
Limiting Reagent Worksheet Answer Key With Work —
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? When dealing with questions where the mol of more than one reactant is. Include work and units for full credit 1. (a) identify the limiting reactant. (hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if.
(B) How Many Moles Of Carbon Dioxide Gas (Co2) Will Form?
Determine the number of moles of al produced. What is a limiting reactant? If 25.4 g of al2o3 is reacted with 10.2 g of fe, determine the limiting reagent. When dealing with questions where the mol of more than one reactant is.
How Many Moles Of Nh3 Can Be Produced From The Reaction Of 28 G Of N2 ?
Include work and units for full credit 1. (hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if you start with 14.8 g of c 3h 8. A student places 2.36 moles of acetic acid. (a) identify the limiting reactant.