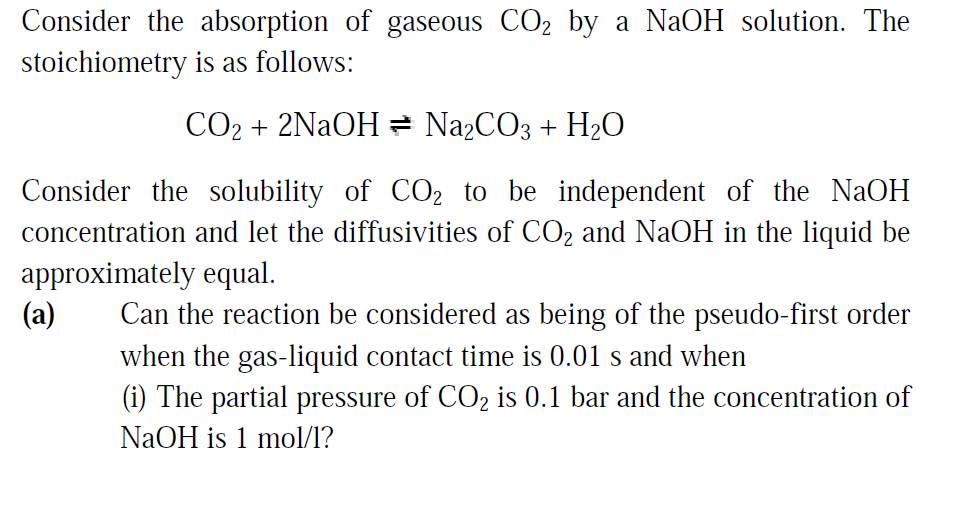Naoh Co2 - Sodium bicarbonate = sodium hydroxide + carbon dioxide. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Balancing of the reaction by hit and trial. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one.
Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Balancing of the reaction by hit and trial. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one.
(a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Balancing of the reaction by hit and trial. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Nahco3 = naoh + co2 is a decomposition reaction where one mole of.
Question Video Deducing the Balanced Chemical Equation for the
Balancing of the reaction by hit and trial. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Nahco3 =.
CO2與NaOH溶液反應後產物判斷問題解析 每日頭條
1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Balancing of the.
Na2CO3+H2O=NaOH+CO2 Balanced EquationSodium carbonate+Water=Sodium
Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number.
NaHCO3 = NaOH + CO2
Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react.
Figure 1 from Changes in CO2 Absorption Efficiency of NaOH Solution
Balancing of the reaction by hit and trial. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Nahco3 = naoh + co2 is a decomposition reaction.
CO2 + NaOH Hấp thụ hoàn toàn 11,2 lít CO2 (đktc) vào dung dịch chứa
Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Balancing of the reaction.
How to Write the Net Ionic Equation for NaOH + CO2 = Na2CO3 + H2O YouTube
Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. 1 naoh + 1.
Consider the absorption of gaseous C02 by a NaOH
Sodium bicarbonate = sodium hydroxide + carbon dioxide. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Balancing of the reaction by hit and trial. 1 naoh + 1 co 2 = 1 na 2.
NaOH+CO2=Na2CO3+H2O. balance the chemical equation mydocumentary838
Balancing of the reaction by hit and trial. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. (a).
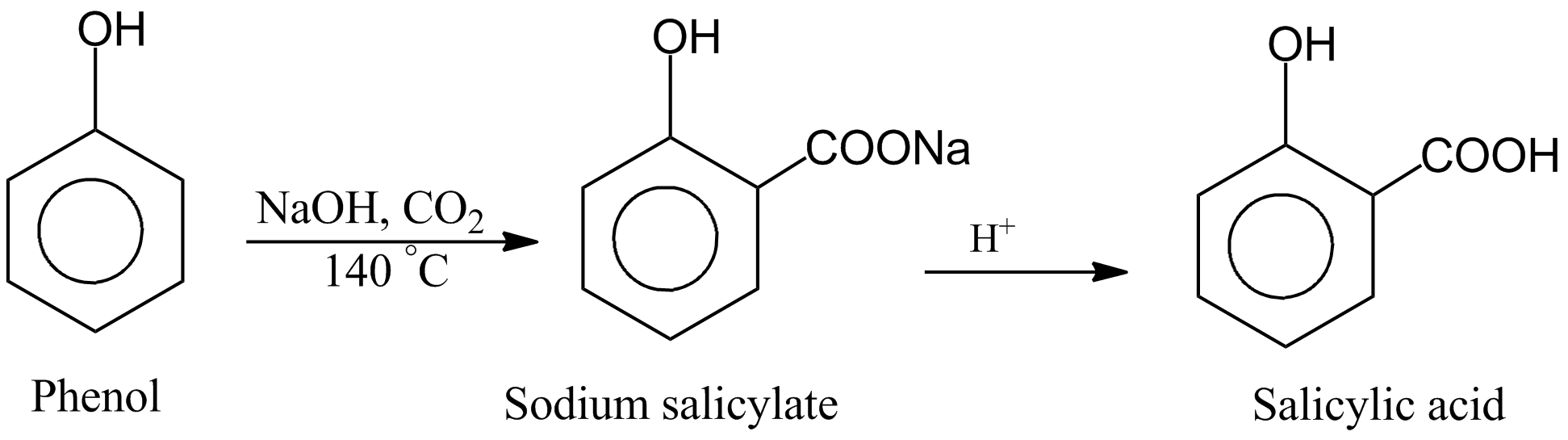
C6H6O + CO2 + NaOH 57ecrass
Sodium bicarbonate = sodium hydroxide + carbon dioxide. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Balancing of the reaction by hit and trial. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check.
(A) When The Alkali ($\Ce{Naoh}$) Solution Is Very Dilute ($\Mathrm{Ph} < 8$), Carbon Dioxide Will First React With Water To Form.
Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation.
Balancing Of The Reaction By Hit And Trial.
Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one.