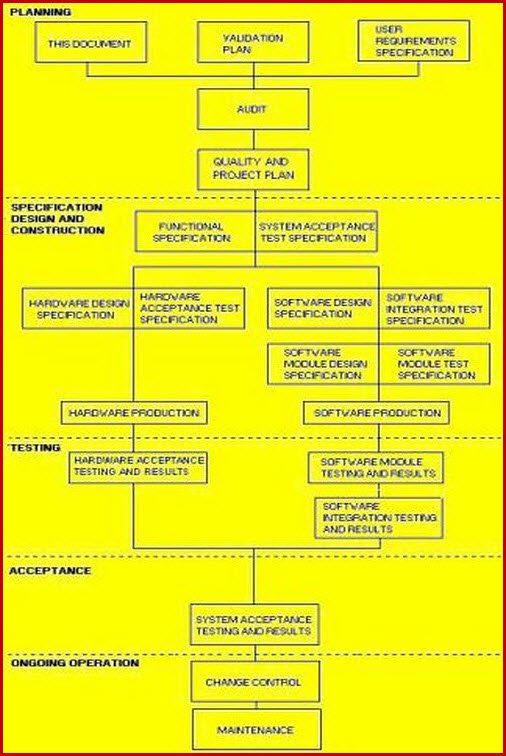
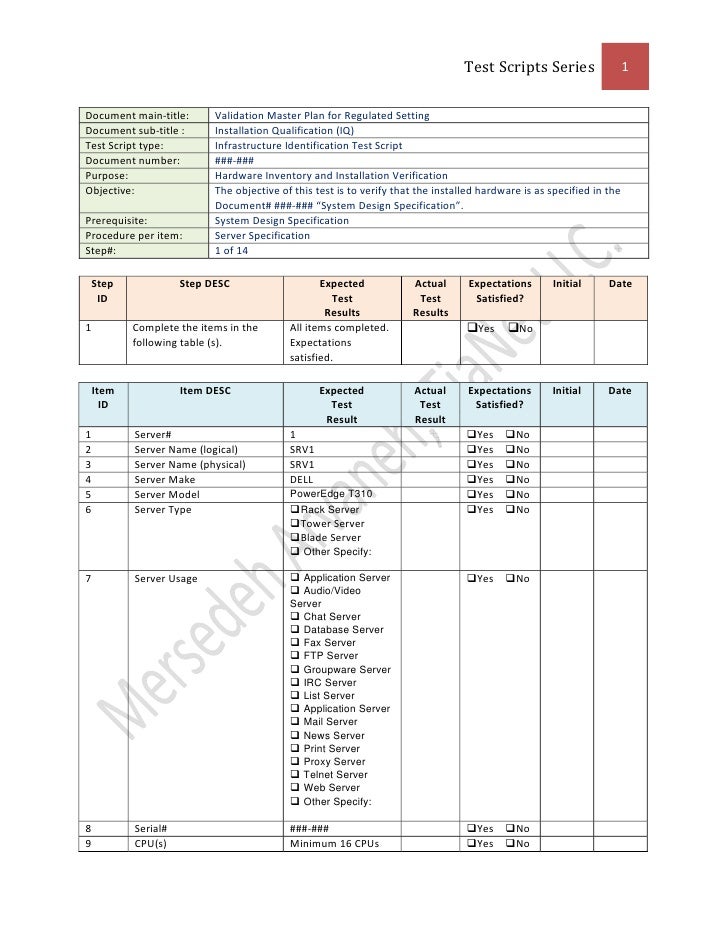
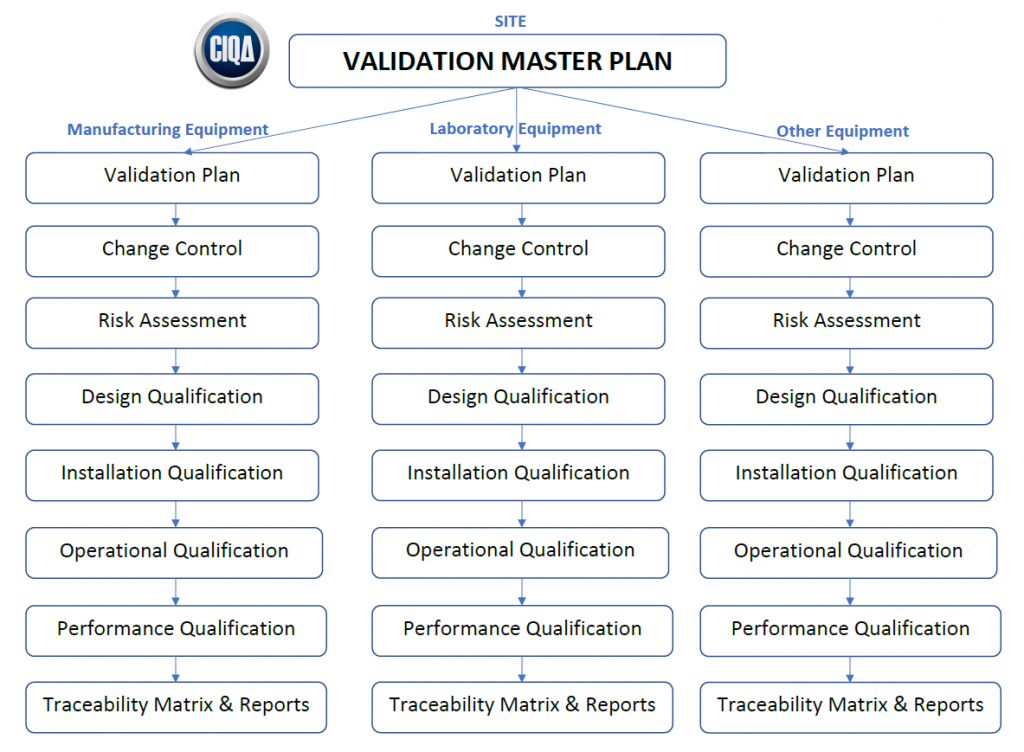
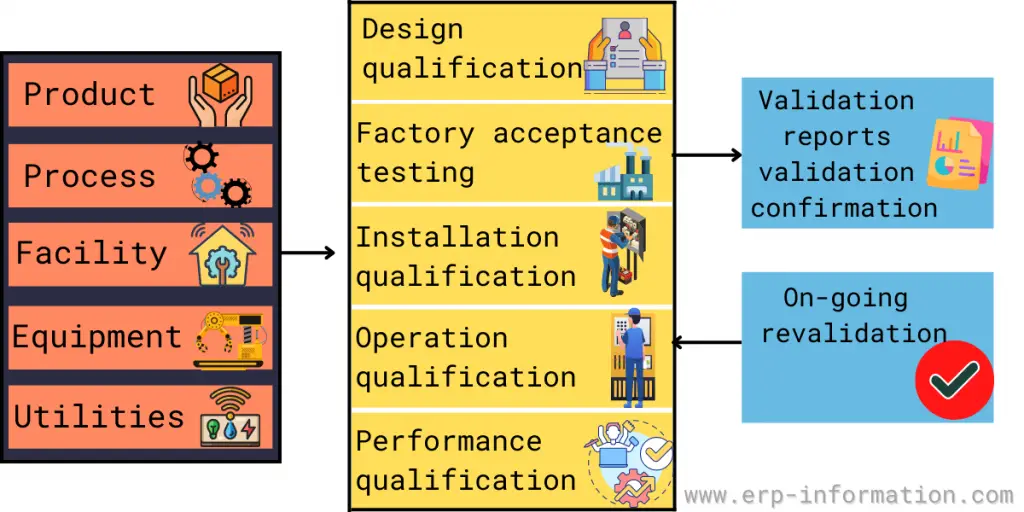
Validation Master Plan Template - The following template is suggested for a validation master plan which can be adapted for local use. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. Items indicated “*” are listed as. This article can help you understand who is responsible for preparing the validation master plan, the stages of the validation life. Describe the purpose of the validation master plan and its scope. Give the location of the facility and define the types of.
Describe the purpose of the validation master plan and its scope. This article can help you understand who is responsible for preparing the validation master plan, the stages of the validation life. The following template is suggested for a validation master plan which can be adapted for local use. Items indicated “*” are listed as. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. Give the location of the facility and define the types of.
Give the location of the facility and define the types of. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. This article can help you understand who is responsible for preparing the validation master plan, the stages of the validation life. Items indicated “*” are listed as. The following template is suggested for a validation master plan which can be adapted for local use. Describe the purpose of the validation master plan and its scope.
Validation Master Plan. Understand the importance and benefits
This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. Items indicated “*” are listed as. Give the location of the facility and define the types of. Describe the purpose of the validation master plan and its scope. This article can help you understand who is responsible for preparing the validation master plan,.
Validation Master Plan (VMP) Downloadable Interactive Template.
Give the location of the facility and define the types of. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. Describe the purpose of the validation master plan and its scope. Items indicated “*” are listed as. The following template is suggested for a validation master plan which can be adapted for.
Validation Master Plan
Give the location of the facility and define the types of. Describe the purpose of the validation master plan and its scope. The following template is suggested for a validation master plan which can be adapted for local use. Items indicated “*” are listed as. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical.
Validation Master Plan Template Validation Center
This article can help you understand who is responsible for preparing the validation master plan, the stages of the validation life. Describe the purpose of the validation master plan and its scope. Items indicated “*” are listed as. The following template is suggested for a validation master plan which can be adapted for local use. This protocol template provides a.
Validation Master Plan Template Verification And Validation
This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. Items indicated “*” are listed as. The following template is suggested for a validation master plan which can be adapted for local use. Describe the purpose of the validation master plan and its scope. This article can help you understand who is responsible.
How to create a Validation Master Plan in 5 steps. Templates & more
This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. Give the location of the facility and define the types of. This article can help you understand who is responsible for preparing the validation master plan, the stages of the validation life. The following template is suggested for a validation master plan which.
Master Validation Plan Template
This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. Describe the purpose of the validation master plan and its scope. Give the location of the facility and define the types of. This article can help you understand who is responsible for preparing the validation master plan, the stages of the validation life..
FREE 9+ Sample Validation Plan Templates in PDF MS Word
The following template is suggested for a validation master plan which can be adapted for local use. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. Describe the purpose of the validation master plan and its scope. This article can help you understand who is responsible for preparing the validation master plan,.
What is Validation Master Plan? (Template, Examples)
Items indicated “*” are listed as. Give the location of the facility and define the types of. This article can help you understand who is responsible for preparing the validation master plan, the stages of the validation life. Describe the purpose of the validation master plan and its scope. This protocol template provides a comprehensive validation master plan (vmp) protocol.
Master Validation Plan PDF
This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. The following template is suggested for a validation master plan which can be adapted for local use. This article can help you understand who is responsible for preparing the validation master plan, the stages of the validation life. Describe the purpose of the.
Items Indicated “*” Are Listed As.
Give the location of the facility and define the types of. This article can help you understand who is responsible for preparing the validation master plan, the stages of the validation life. Describe the purpose of the validation master plan and its scope. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies.