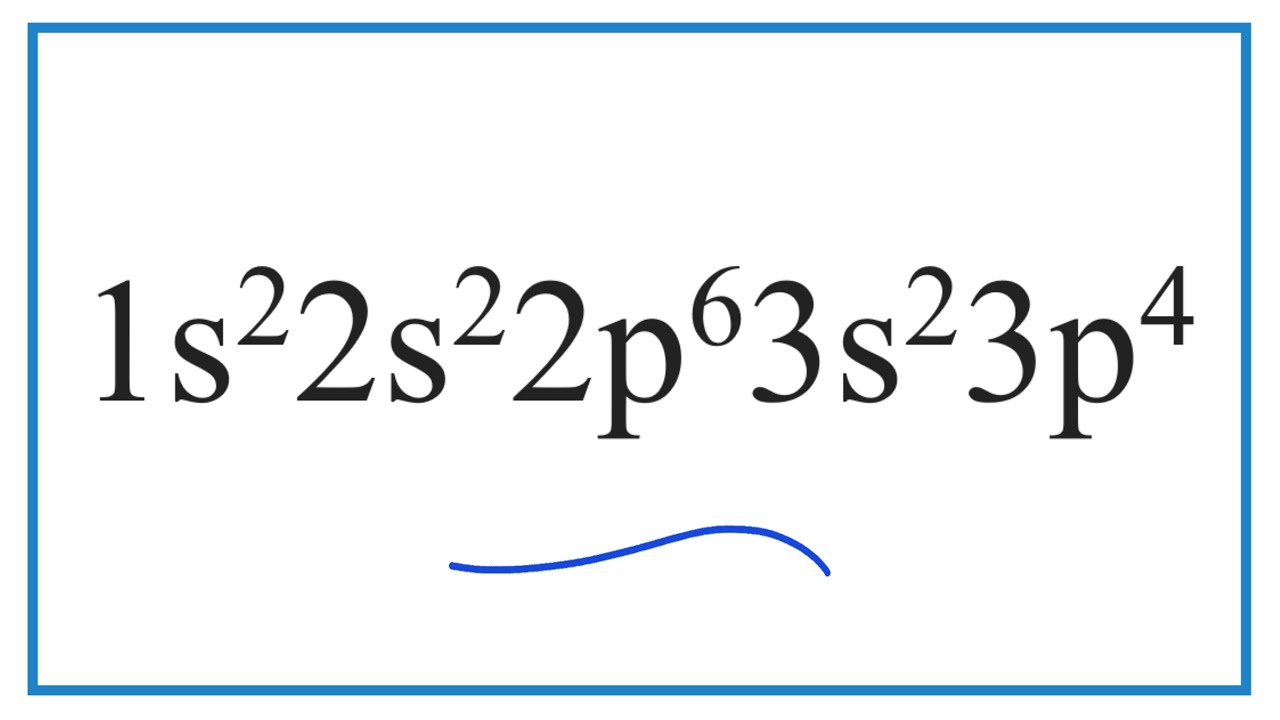What Element Has The Electron Configuration 1S22S22P63S23P4 - In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. Cations and anions increase from. Therefore, the atomic number 16 denotes that the element is. What is characteristic of the electron configurations of noble gases? According to the given electronic configuration the total number of electrons is 16.
According to the given electronic configuration the total number of electrons is 16. In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. What is characteristic of the electron configurations of noble gases? Therefore, the atomic number 16 denotes that the element is. Cations and anions increase from.
In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. Therefore, the atomic number 16 denotes that the element is. What is characteristic of the electron configurations of noble gases? According to the given electronic configuration the total number of electrons is 16. Cations and anions increase from.
Solved Click in the answer box to activate the palette.
According to the given electronic configuration the total number of electrons is 16. What is characteristic of the electron configurations of noble gases? Therefore, the atomic number 16 denotes that the element is. Cations and anions increase from. In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which.
Printable Periodic Table Of Elements With Electron Configuration
In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. Cations and anions increase from. What is characteristic of the electron configurations of noble gases? According to the given electronic configuration the total number of electrons is 16. Therefore, the atomic number 16 denotes that the element is.
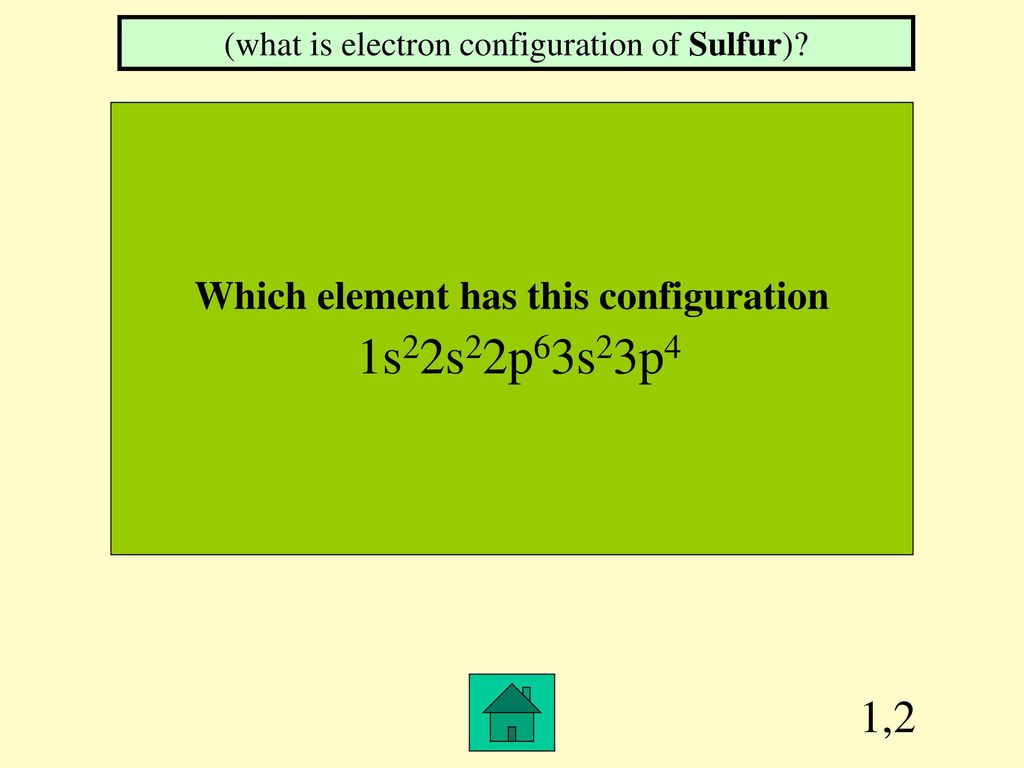
Jeopardy Hosted by Chem Istry. ppt download
Cations and anions increase from. In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. Therefore, the atomic number 16 denotes that the element is. According to the given electronic configuration the total number of electrons is 16. What is characteristic of the electron configurations of noble gases?
What Element Has the Electron Configuration 1s22s22p63s23p3
In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. Cations and anions increase from. According to the given electronic configuration the total number of electrons is 16. Therefore, the atomic number 16 denotes that the element is. What is characteristic of the electron configurations of noble gases?
Solved 1. Consider the element with the electron
According to the given electronic configuration the total number of electrons is 16. Therefore, the atomic number 16 denotes that the element is. Cations and anions increase from. In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. What is characteristic of the electron configurations of noble gases?
PPT Ch. 13 Quantum Mechanical Model PowerPoint Presentation, free
In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. Therefore, the atomic number 16 denotes that the element is. Cations and anions increase from. What is characteristic of the electron configurations of noble gases? According to the given electronic configuration the total number of electrons is 16.
SOLVED l. If one electron is added to the outer shell of chlorine,it
What is characteristic of the electron configurations of noble gases? Cations and anions increase from. Therefore, the atomic number 16 denotes that the element is. In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. According to the given electronic configuration the total number of electrons is 16.
Solved 1. What element has the electron configuration
Therefore, the atomic number 16 denotes that the element is. According to the given electronic configuration the total number of electrons is 16. In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. Cations and anions increase from. What is characteristic of the electron configurations of noble gases?
[Solved] Which element has the following electron configuration
Cations and anions increase from. In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. Therefore, the atomic number 16 denotes that the element is. What is characteristic of the electron configurations of noble gases? According to the given electronic configuration the total number of electrons is 16.
Electronic Configuration Antimony Learn Important Terms and Concepts
Therefore, the atomic number 16 denotes that the element is. What is characteristic of the electron configurations of noble gases? According to the given electronic configuration the total number of electrons is 16. In the given electron configuration, 1s22s22p63s23p4, the atom's valence electrons are those in the outermost energy level, which. Cations and anions increase from.
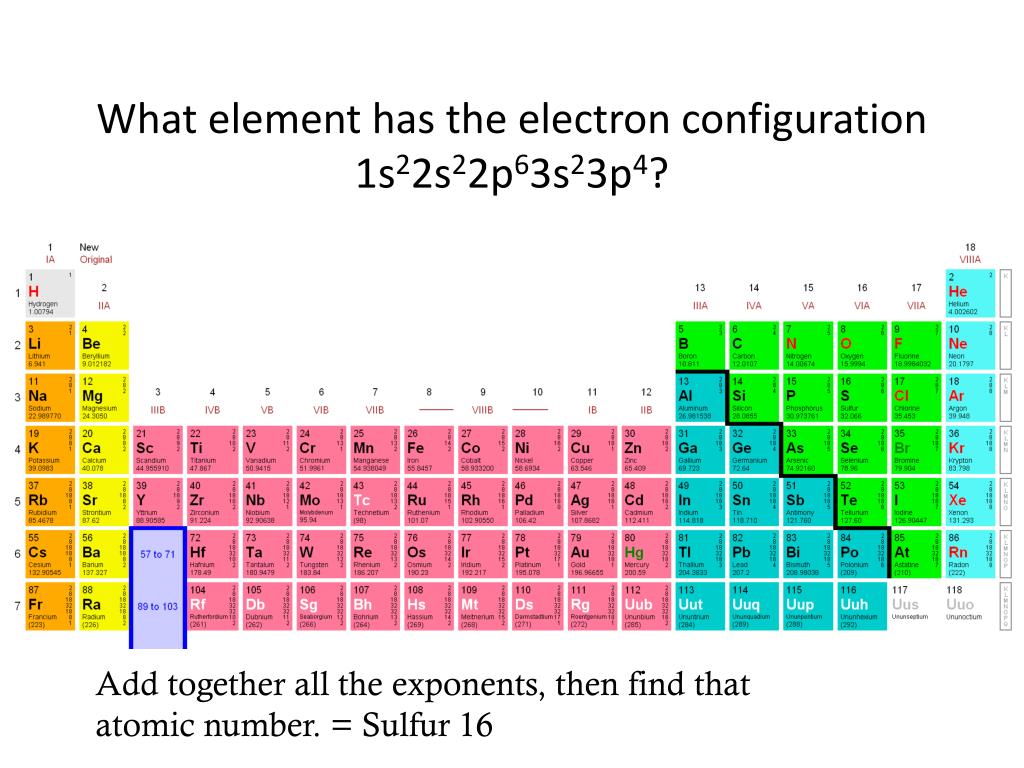
In The Given Electron Configuration, 1S22S22P63S23P4, The Atom's Valence Electrons Are Those In The Outermost Energy Level, Which.
Therefore, the atomic number 16 denotes that the element is. Cations and anions increase from. According to the given electronic configuration the total number of electrons is 16. What is characteristic of the electron configurations of noble gases?