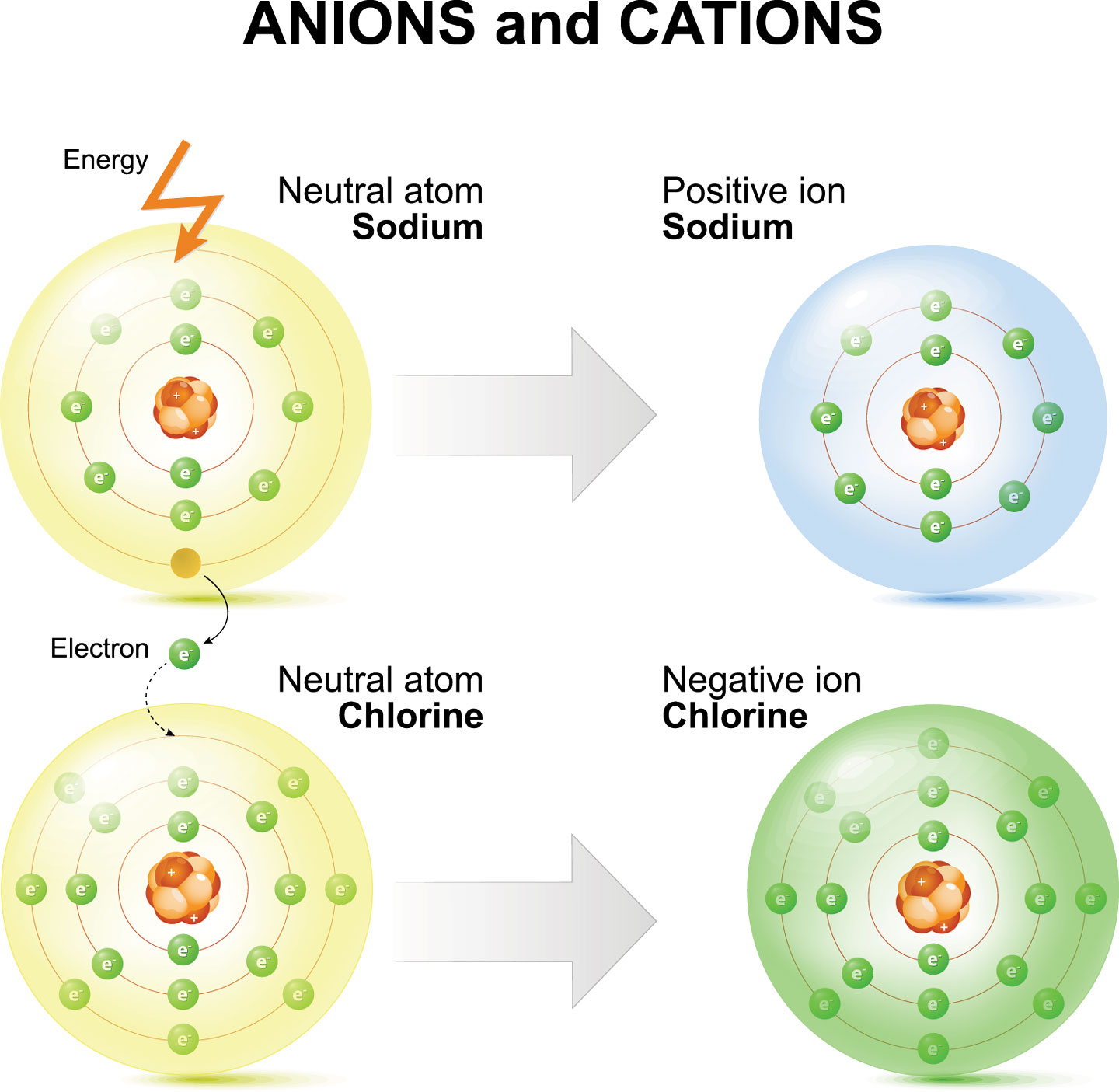
What Ion Does Chlorine Form - There are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Chlorine forms an ion by gaining one electron to achieve a stable electron configuration.
Chlorine forms an ion by gaining one electron to achieve a stable electron configuration. There are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of.
There are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Chlorine forms an ion by gaining one electron to achieve a stable electron configuration.
Chlorine Periodic Table and Atomic Properties
Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Chlorine forms an ion by gaining one electron to achieve a stable electron configuration. There are two chloride ions in the formula.
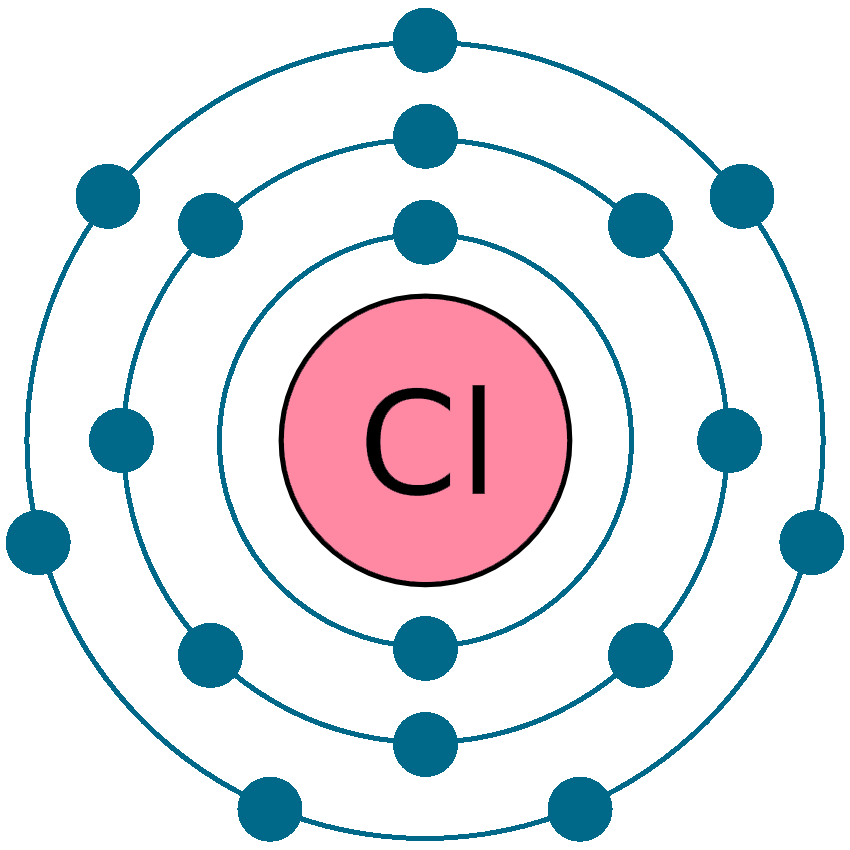
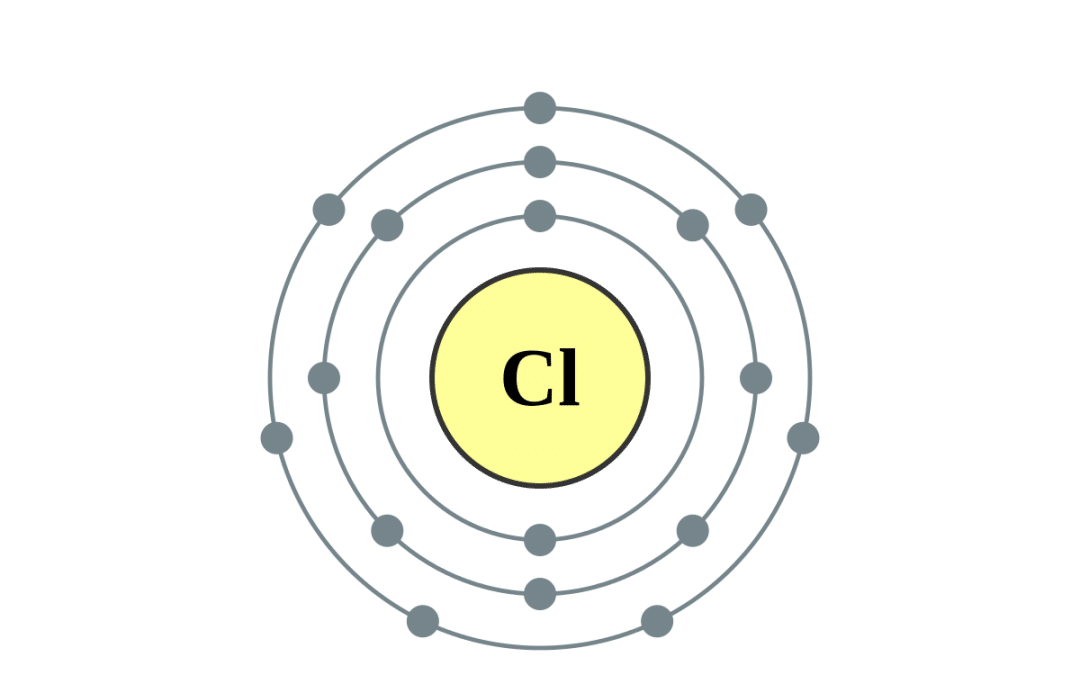
Draw the atomic structure of the Chlorine atom and chlorine ion
There are two chloride ions in the formula. Chlorine forms an ion by gaining one electron to achieve a stable electron configuration. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of.
Explainer Ions and radicals in our world Science News for Students
Chlorine forms an ion by gaining one electron to achieve a stable electron configuration. There are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of.
compound,element,and mixtures John's Blog
Chlorine forms an ion by gaining one electron to achieve a stable electron configuration. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. There are two chloride ions in the formula.
Aufbau Diagram For Chlorine
Chlorine forms an ion by gaining one electron to achieve a stable electron configuration. There are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of.
chlorine Uses, Properties, & Facts Britannica
Chlorine forms an ion by gaining one electron to achieve a stable electron configuration. There are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of.
Chlorine Cl (Element 17) of Periodic Table Newton Desk
Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Chlorine forms an ion by gaining one electron to achieve a stable electron configuration. There are two chloride ions in the formula.
What Does It Mean When An Ion Has A Positive Charge Design Talk
Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Chlorine forms an ion by gaining one electron to achieve a stable electron configuration. There are two chloride ions in the formula.
15 Interesting Facts About Chlorine
There are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is not part of. Chlorine forms an ion by gaining one electron to achieve a stable electron configuration.
Although Chlorine As An Element Is A Diatomic Molecule, Cl 2, Elemental Chlorine Is Not Part Of.
There are two chloride ions in the formula. Chlorine forms an ion by gaining one electron to achieve a stable electron configuration.