What Is The Element With An Electron Configuration Of 1S22S22P63S23P64S23D5 - 119 rows the electron configuration shows the distribution of electrons into subshells. Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. This list of electron configurations of elements. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn).
119 rows the electron configuration shows the distribution of electrons into subshells. Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. This list of electron configurations of elements. Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23.
To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. 119 rows the electron configuration shows the distribution of electrons into subshells. Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. This list of electron configurations of elements. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn).
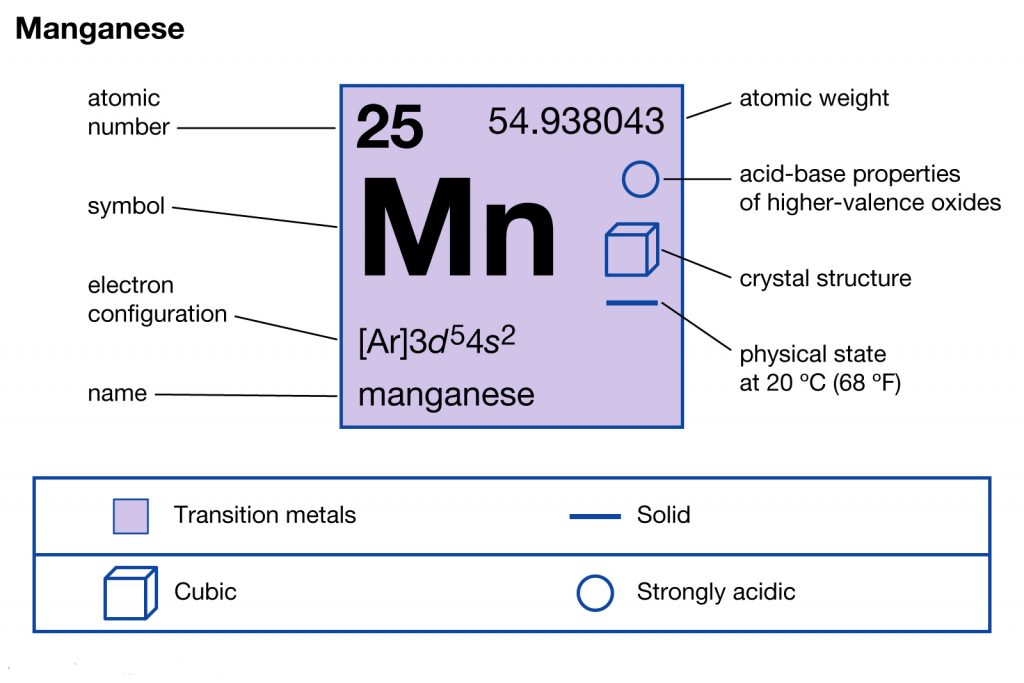
Manganese Electron Configuration (Mn) with Orbital Diagram
119 rows the electron configuration shows the distribution of electrons into subshells. To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. This list of electron configurations of elements. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium.
Electron configuration of every element in the periodic table
This list of electron configurations of elements. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). 119 rows the electron configuration shows the distribution of electrons into subshells. Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. Study with quizlet and memorize flashcards containing terms like.
(1) The element with an electron configuration of 1s22s22p63s23p64s23d5
To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. This list of electron configurations of elements. 119 rows the electron configuration shows the distribution of.
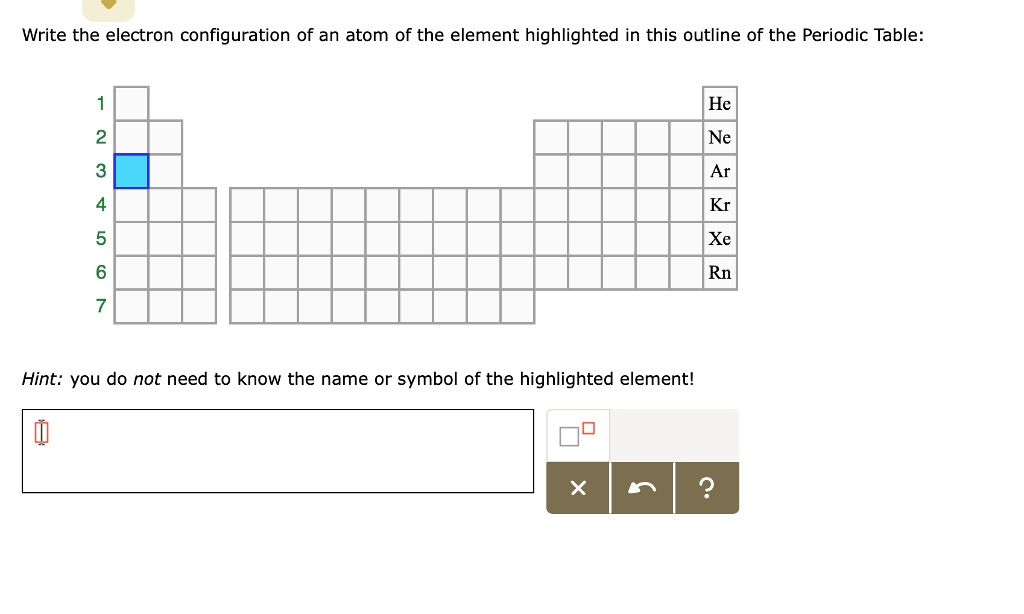
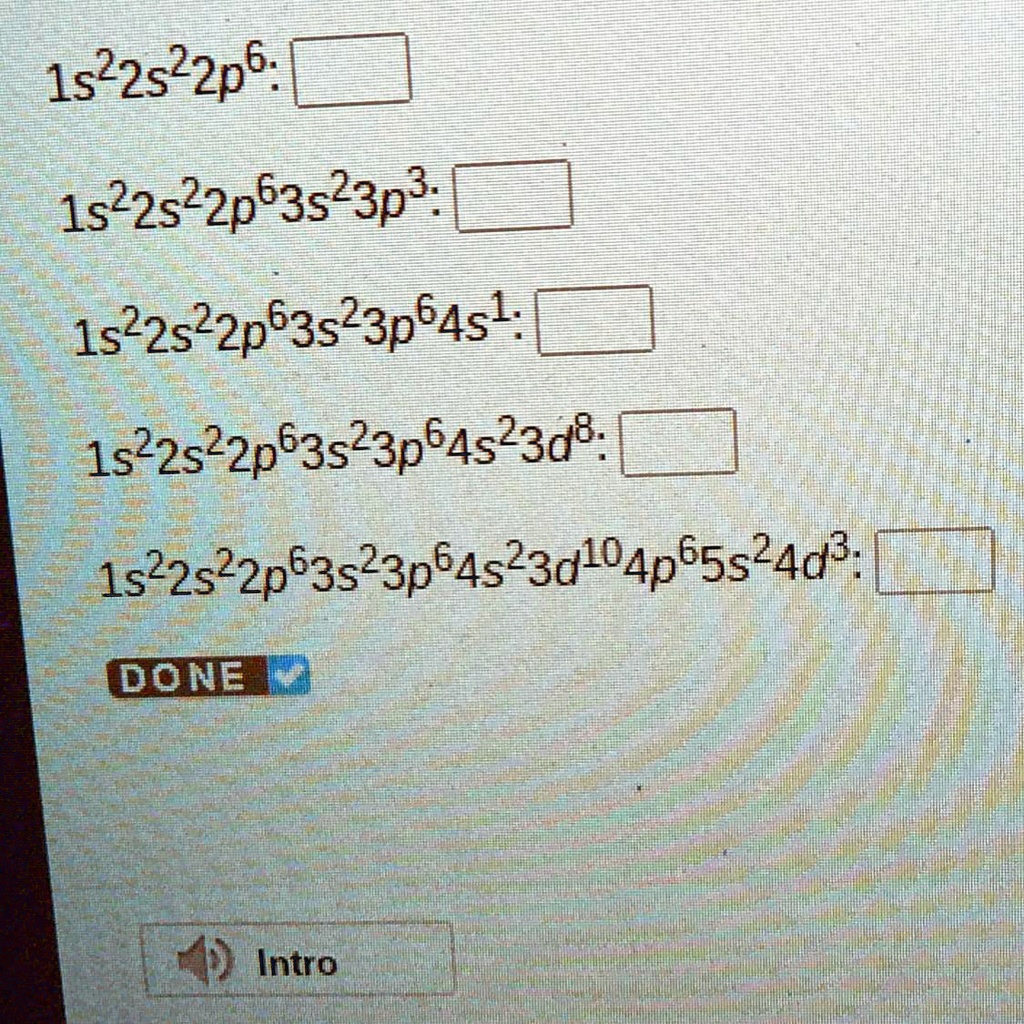
write the electron configuration of an atom of the element highlighted
119 rows the electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. Study with.
1.5 Electronic Structure of Atoms (Electron Configurations)
This list of electron configurations of elements. 119 rows the electron configuration shows the distribution of electrons into subshells. To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). Study with quizlet and memorize flashcards containing terms like.
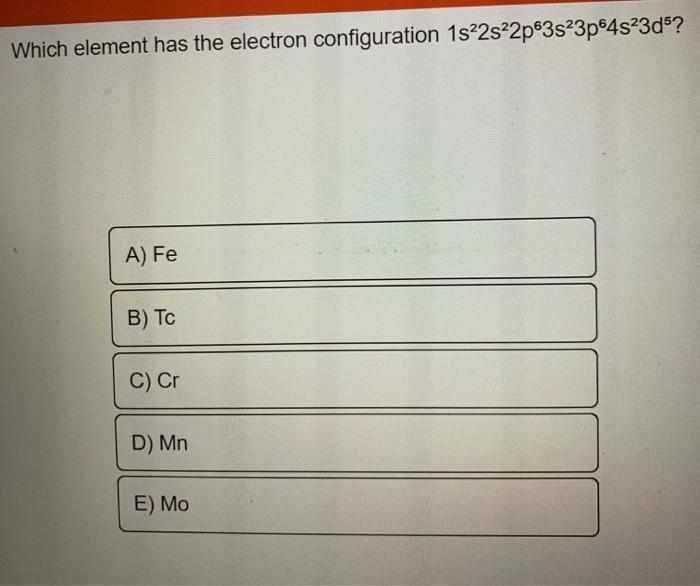
Solved Which element has the electron configuration
To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. 119 rows the electron configuration shows the distribution of electrons into subshells. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. Study with quizlet and memorize flashcards containing terms like.
Electron Configuration of Elements Chemistry Periodic Table
The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). This list of electron configurations of elements. Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. To identify the element.
use the periodic table to identify the element indicated by each
The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. 119 rows the electron configuration shows the distribution of electrons into subshells. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of.
Electronic Configuration Antimony Learn Important Terms and Concepts
The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). This list of electron configurations of elements. Study with quizlet and memorize flashcards.
[Solved] Which element has the following electron configuration
To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. This list of electron configurations of elements. 119 rows the electron configuration shows the distribution of electrons into subshells. Study with.
To Identify The Element With The Electron Configuration , First Count The Total Number Of Electrons By Adding The Exponents Of The.
This list of electron configurations of elements. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. 119 rows the electron configuration shows the distribution of electrons into subshells. Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar.
The Electron Configuration 1S22S22P63S23P64S23D5 Corresponds To The Element Manganese (Mn).
Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an.