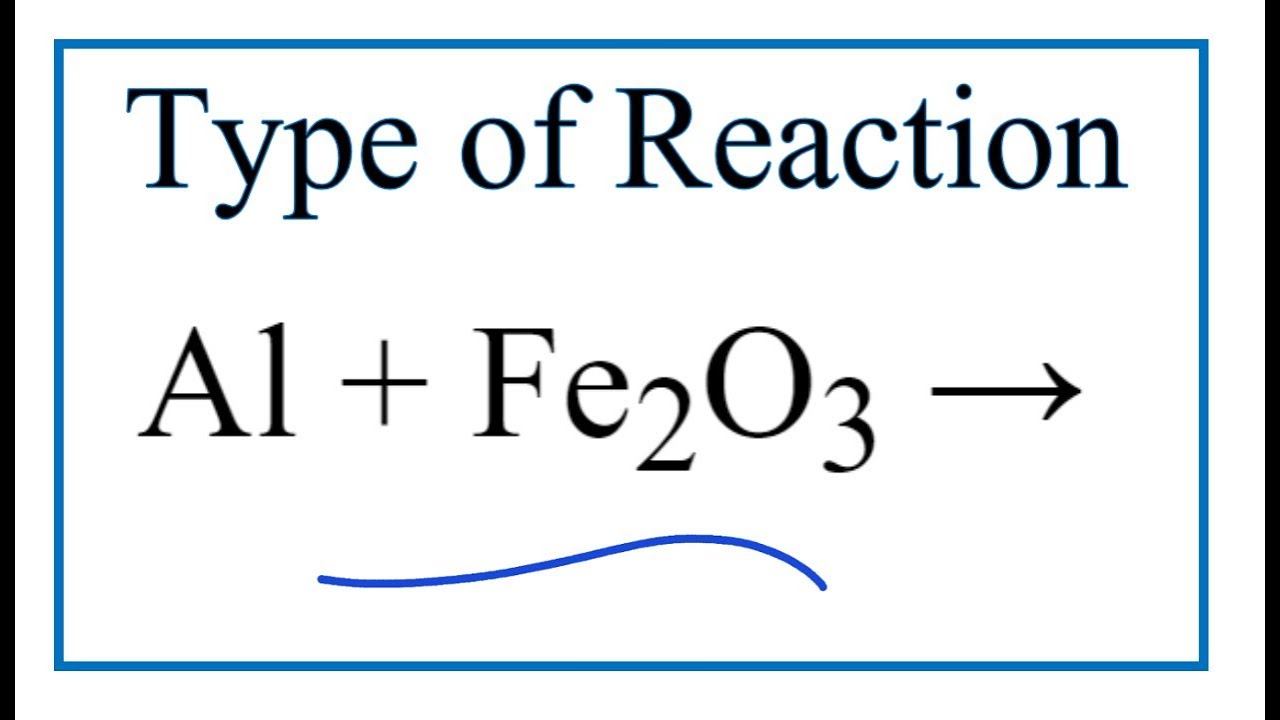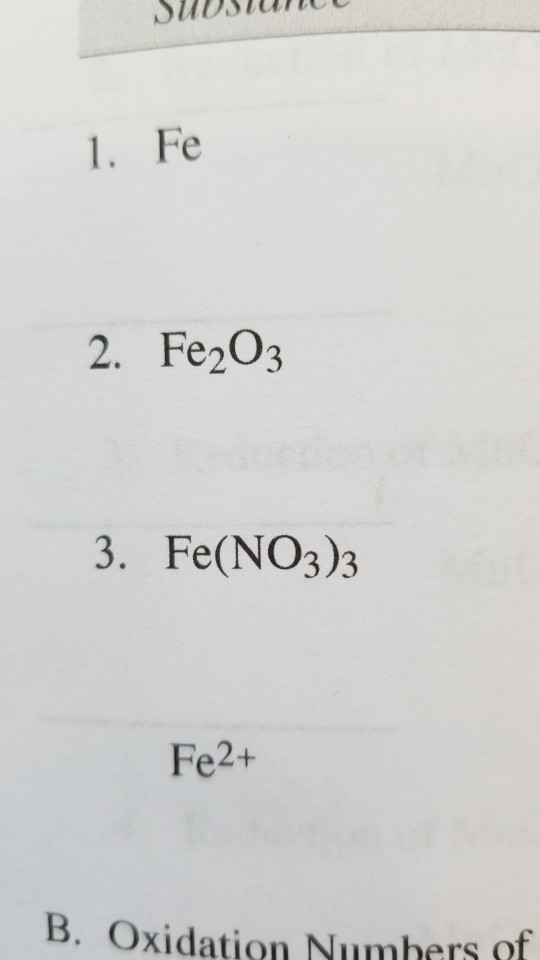What Is The Oxidation Number Of Iron In Fe2O3 - Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The oxidation state of fe 2 o 3 is. The bond between the oxygen and iron atoms is an ionic bond, resulting from the difference in. This means that each fe atom. The oxidation state of iron(iii) oxide is +3. The (ii) and (iii) are the oxidation states of the iron in the two compounds: That tells you that they contain fe 2.
The oxidation state of fe 2 o 3 is. The (ii) and (iii) are the oxidation states of the iron in the two compounds: The bond between the oxygen and iron atoms is an ionic bond, resulting from the difference in. This means that each fe atom. The oxidation state of iron(iii) oxide is +3. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. That tells you that they contain fe 2.
That tells you that they contain fe 2. The (ii) and (iii) are the oxidation states of the iron in the two compounds: The bond between the oxygen and iron atoms is an ionic bond, resulting from the difference in. The oxidation state of iron(iii) oxide is +3. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. This means that each fe atom. The oxidation state of fe 2 o 3 is.
how to calculate oxidation number of fe in feso4 (nh4)2so4 6h2o
The oxidation state of fe 2 o 3 is. The oxidation state of iron(iii) oxide is +3. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The (ii) and (iii) are the oxidation states of the iron in the two compounds: That tells you that they contain fe 2.
Oxidation number of Fe (Iron) in Fe3O4// Redox reaction
Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The oxidation state of iron(iii) oxide is +3. The oxidation state of fe 2 o 3 is. That tells you that they contain fe 2. The (ii) and (iii) are the oxidation states of the iron in the two compounds:
What Is the Oxidation Number of Iron in Fe2o3 TalonwellFritz
The bond between the oxygen and iron atoms is an ionic bond, resulting from the difference in. This means that each fe atom. The (ii) and (iii) are the oxidation states of the iron in the two compounds: The oxidation state of iron(iii) oxide is +3. The oxidation state of fe 2 o 3 is.
For oxidation of iron,4Fe(s)+3O2 (g)→2Fe2 O3 (s)entropy change is 549
The oxidation state of fe 2 o 3 is. This means that each fe atom. The (ii) and (iii) are the oxidation states of the iron in the two compounds: The bond between the oxygen and iron atoms is an ionic bond, resulting from the difference in. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has.
Type of Reaction for Al + Fe2O3 = Al2O3 + Fe YouTube
This means that each fe atom. The oxidation state of iron(iii) oxide is +3. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The (ii) and (iii) are the oxidation states of the iron in the two compounds: The oxidation state of fe 2 o 3 is.
Solved 1. Fe 2. Fe2O3 3. Fe(NO3)3 Fe2+ B. Oxidation Numbers
Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The (ii) and (iii) are the oxidation states of the iron in the two compounds: That tells you that they contain fe 2. The oxidation state of fe 2 o 3 is. The bond between the oxygen and iron atoms.
Calculate the OXIDATION NUMBER of Iron (Fe) in Fe3O4 YouTube
The (ii) and (iii) are the oxidation states of the iron in the two compounds: This means that each fe atom. The bond between the oxygen and iron atoms is an ionic bond, resulting from the difference in. The oxidation state of iron(iii) oxide is +3. That tells you that they contain fe 2.
Fe2o3 Oxidation Number
This means that each fe atom. That tells you that they contain fe 2. The oxidation state of fe 2 o 3 is. The bond between the oxygen and iron atoms is an ionic bond, resulting from the difference in. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms.
Answered What is the oxidation number of iron,… bartleby
This means that each fe atom. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The oxidation state of fe 2 o 3 is. The (ii) and (iii) are the oxidation states of the iron in the two compounds: The bond between the oxygen and iron atoms is an.
IRON OXIDE Iron (III) Oxide Minium Pigments and Stains
The (ii) and (iii) are the oxidation states of the iron in the two compounds: This means that each fe atom. That tells you that they contain fe 2. The oxidation state of iron(iii) oxide is +3. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms.
Fe 2 O 3 Is The Chemical Formula Of Iron(Iii) Oxide Which Has Three Oxygen Atoms, And Two Iron Atoms.
The (ii) and (iii) are the oxidation states of the iron in the two compounds: This means that each fe atom. The oxidation state of fe 2 o 3 is. That tells you that they contain fe 2.
The Oxidation State Of Iron(Iii) Oxide Is +3.
The bond between the oxygen and iron atoms is an ionic bond, resulting from the difference in.