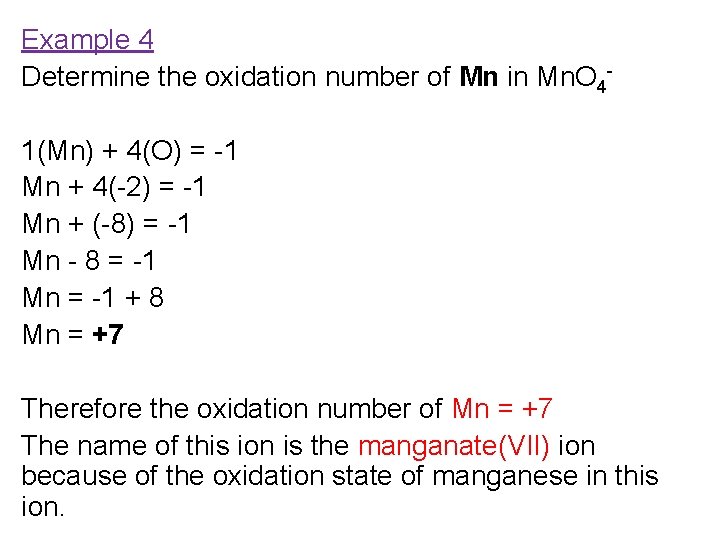
What Is The Oxidation Number Of Manganese In Mno4 - Let's break down the reasoning behind this. In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by four oxygen (o) atoms. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Therefore the oxidation state of mn. The oxidation number of manganese (mn) in the permanganate ion (mno₄⁻) is indeed +7.
In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by four oxygen (o) atoms. Let's break down the reasoning behind this. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Therefore the oxidation state of mn. The oxidation number of manganese (mn) in the permanganate ion (mno₄⁻) is indeed +7.
Therefore the oxidation state of mn. In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by four oxygen (o) atoms. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. The oxidation number of manganese (mn) in the permanganate ion (mno₄⁻) is indeed +7. Let's break down the reasoning behind this.
What is the oxidation number of the manganese atom (Mn) in this
In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by four oxygen (o) atoms. Therefore the oxidation state of mn. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. The oxidation number of manganese (mn) in the permanganate ion (mno₄⁻) is indeed +7. Let's break down.
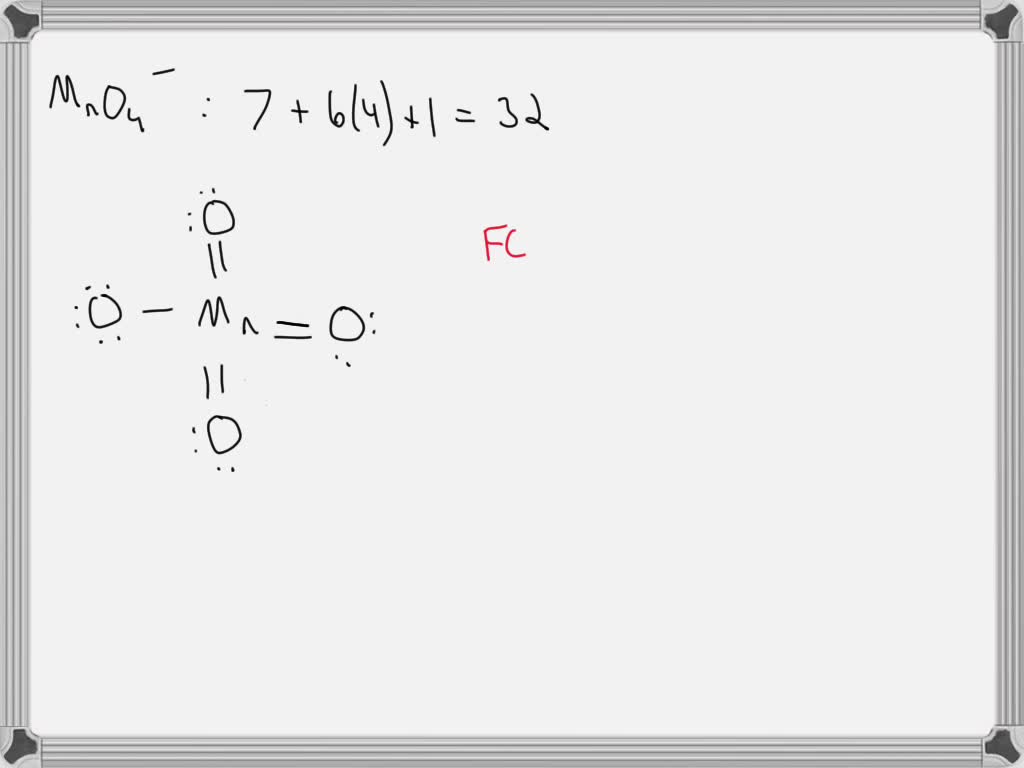
SOLVED Draw the Lewis structure for the permanganate ion MnO4 and
So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by four oxygen (o) atoms. Therefore the oxidation state of mn. The oxidation number of manganese (mn) in the permanganate ion (mno₄⁻) is indeed +7. Let's break down.
Oxidation Number of Mn in Mno4
In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by four oxygen (o) atoms. Let's break down the reasoning behind this. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Therefore the oxidation state of mn. The oxidation number of manganese (mn) in the permanganate ion.
SOLVEDCalculate the oxidation number of manganese in the following
Let's break down the reasoning behind this. The oxidation number of manganese (mn) in the permanganate ion (mno₄⁻) is indeed +7. Therefore the oxidation state of mn. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by.
Oxidation Number of Mn in Mno4
Let's break down the reasoning behind this. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by four oxygen (o) atoms. Therefore the oxidation state of mn. The oxidation number of manganese (mn) in the permanganate ion.
SOLVED Manganese has the oxidation number of +7 in OA [MnF6)]3. B
So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Let's break down the reasoning behind this. The oxidation number of manganese (mn) in the permanganate ion (mno₄⁻) is indeed +7. Therefore the oxidation state of mn. In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by.
Oxidation Number for MnO4 YouTube
In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by four oxygen (o) atoms. Therefore the oxidation state of mn. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. The oxidation number of manganese (mn) in the permanganate ion (mno₄⁻) is indeed +7. Let's break down.
Oxidationreduction reactions Oxidation and reduction oxygen transer A
The oxidation number of manganese (mn) in the permanganate ion (mno₄⁻) is indeed +7. Let's break down the reasoning behind this. In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by four oxygen (o) atoms. Therefore the oxidation state of mn. So, the oxidation number of manganese (mn) in potassium permanganate mno.
Manganese compounds colors (different oxidation states) chemistry
The oxidation number of manganese (mn) in the permanganate ion (mno₄⁻) is indeed +7. In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by four oxygen (o) atoms. Let's break down the reasoning behind this. Therefore the oxidation state of mn. So, the oxidation number of manganese (mn) in potassium permanganate mno.
7. Calculate the oxidation number of Mn in KMnO4
In the permanganate ion (m n o 4 −), the manganese (m n) atom is tetrahedrally surrounded by four oxygen (o) atoms. Let's break down the reasoning behind this. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4. Therefore the oxidation state of mn. The oxidation number of manganese (mn) in the permanganate ion.
In The Permanganate Ion (M N O 4 −), The Manganese (M N) Atom Is Tetrahedrally Surrounded By Four Oxygen (O) Atoms.
Let's break down the reasoning behind this. Therefore the oxidation state of mn. The oxidation number of manganese (mn) in the permanganate ion (mno₄⁻) is indeed +7. So, the oxidation number of manganese (mn) in potassium permanganate mno 2 is + 4.




![SOLVED Manganese has the oxidation number of +7 in OA [MnF6)]3. B](https://cdn.numerade.com/ask_images/eefa9df997f84c96ba55933c7a65b854.jpg)