What Is The Product Of Hcl Naoh - The type of salt produced depends on the. The product of neutralization is a salt and water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible.
The type of salt produced depends on the. The product of neutralization is a salt and water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible.
A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible. The product of neutralization is a salt and water. The type of salt produced depends on the.
Enthalpy of neutralization for both HNO3 and HCl with NaOH is 57.1kJ
The product of neutralization is a salt and water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible. The type of salt produced depends on the.
20ML of 1N HCl reacted with 30ml of 0.5N Naoh, the normality of the
The product of neutralization is a salt and water. The type of salt produced depends on the. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible.
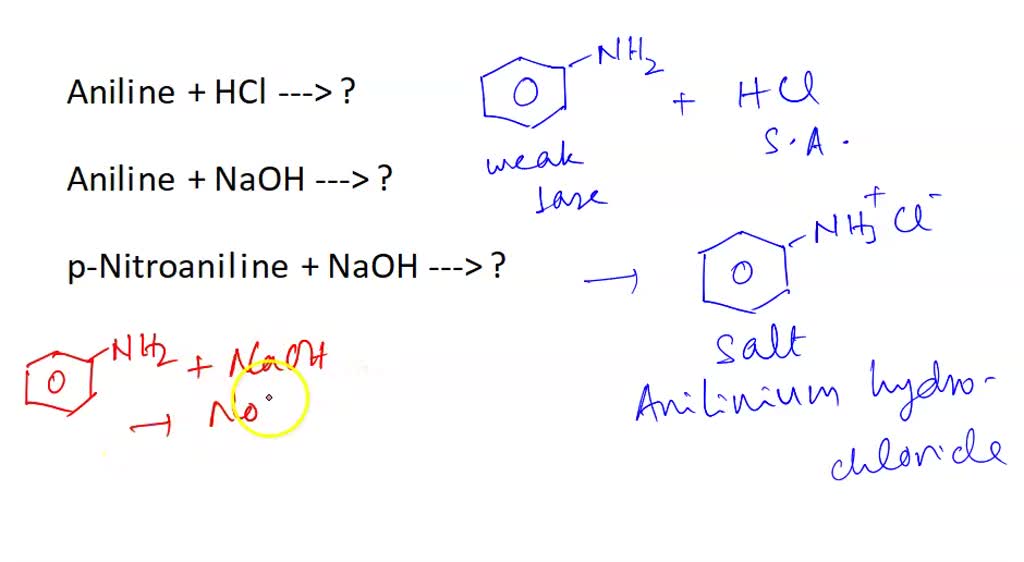
SOLVED Aniline + HCl —> ? Aniline + NaOH —> ? pNitroaniline + NaOH —> ?
A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible. The product of neutralization is a salt and water. The type of salt produced depends on the.
Acid/Base Neutralization Reaction for NaOH + HCl (Sodium hydroxide
A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible. The product of neutralization is a salt and water. The type of salt produced depends on the.
0 1 N Naoh Preparation How To Prepare And Standardize 0 1 N Sodium
The type of salt produced depends on the. The product of neutralization is a salt and water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible.
TJD HCL TJD Shop
The product of neutralization is a salt and water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible. The type of salt produced depends on the.
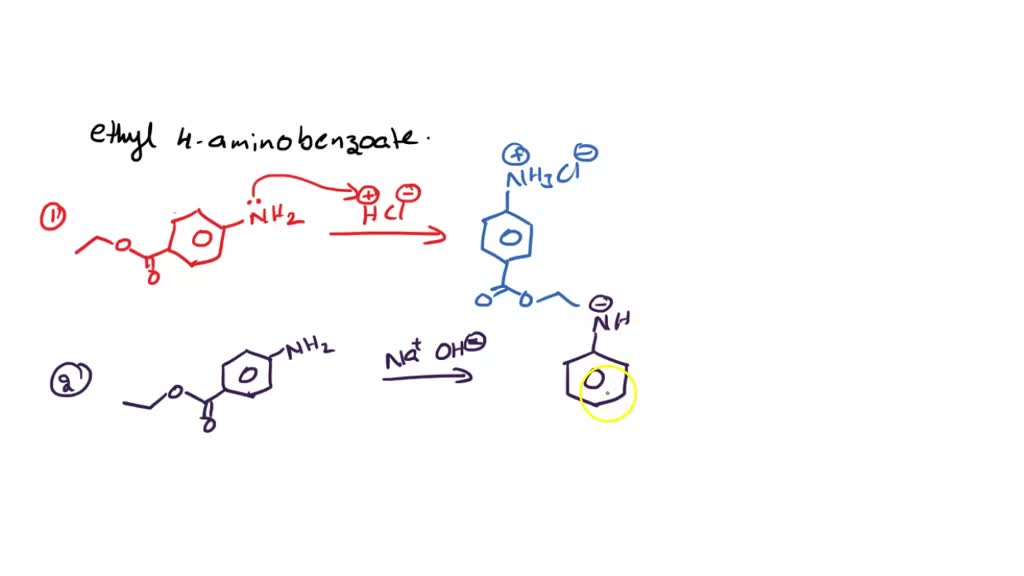
What are the reactions for ethyl 4aminobenzoate + HCl > ethyl 4
The product of neutralization is a salt and water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible. The type of salt produced depends on the.
HOW TO ACCEPT HCL OFFER LETTER hcl offer letter hcl onboarding
A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible. The product of neutralization is a salt and water. The type of salt produced depends on the.
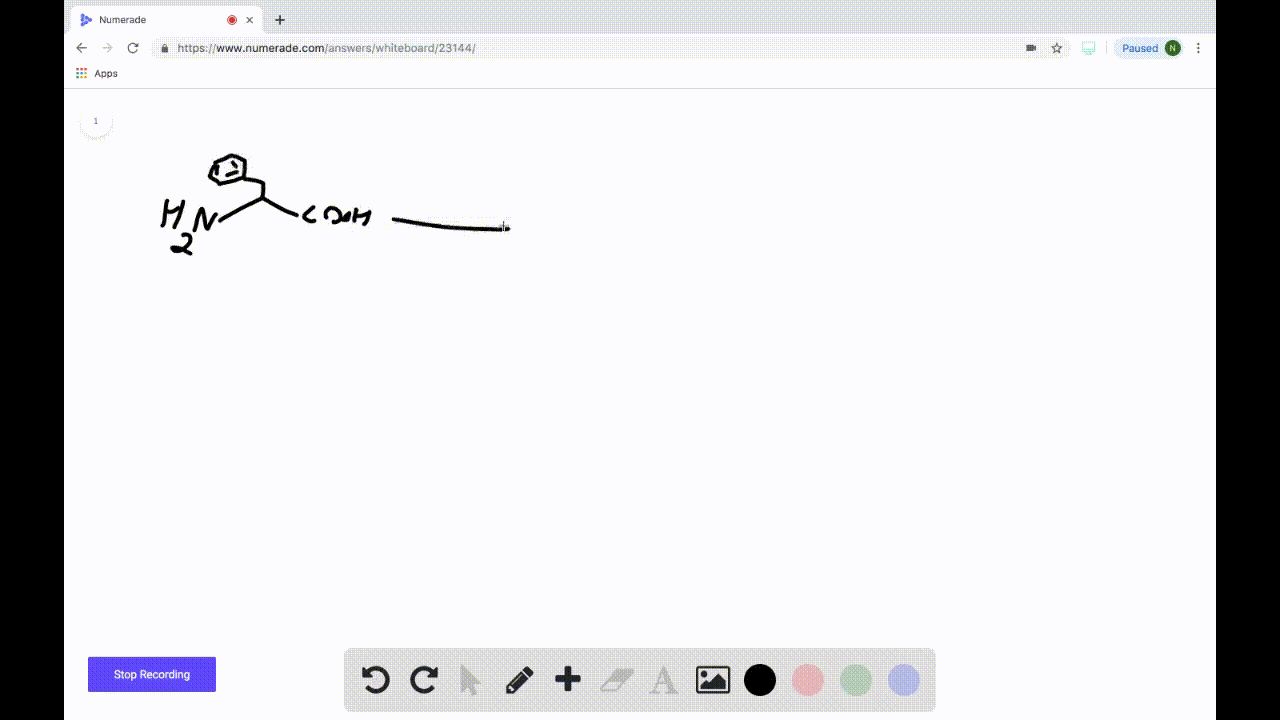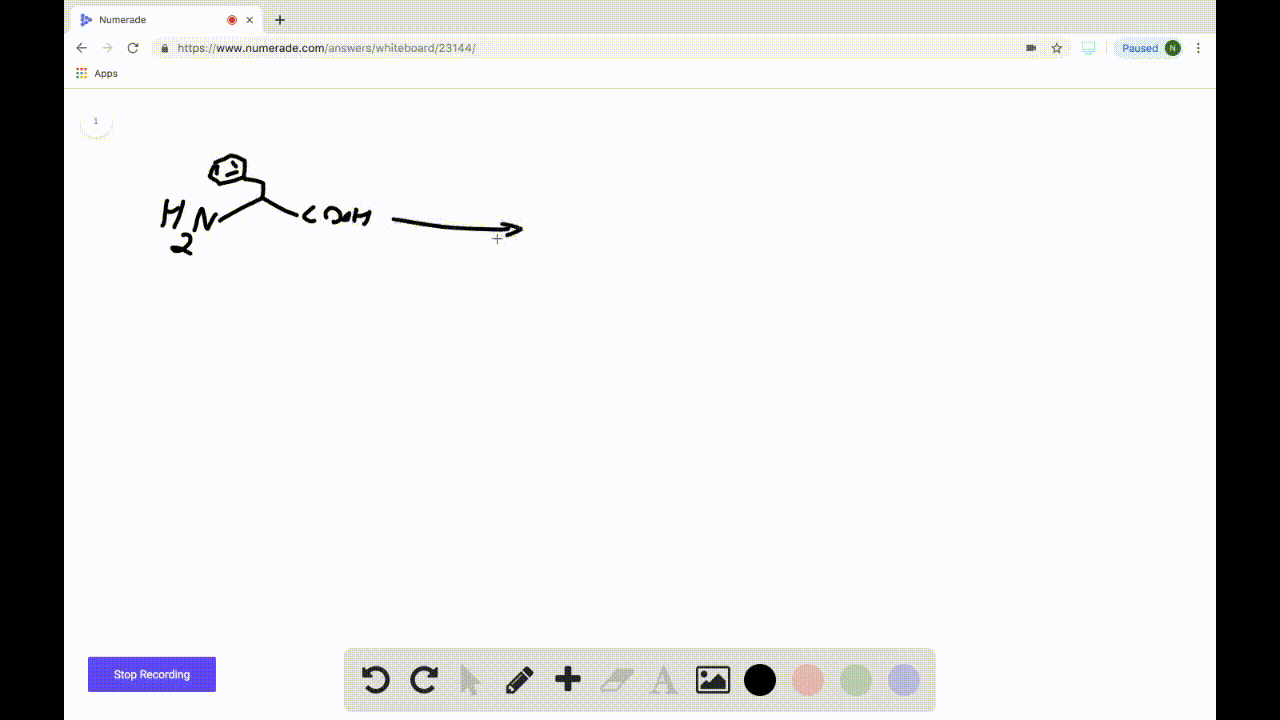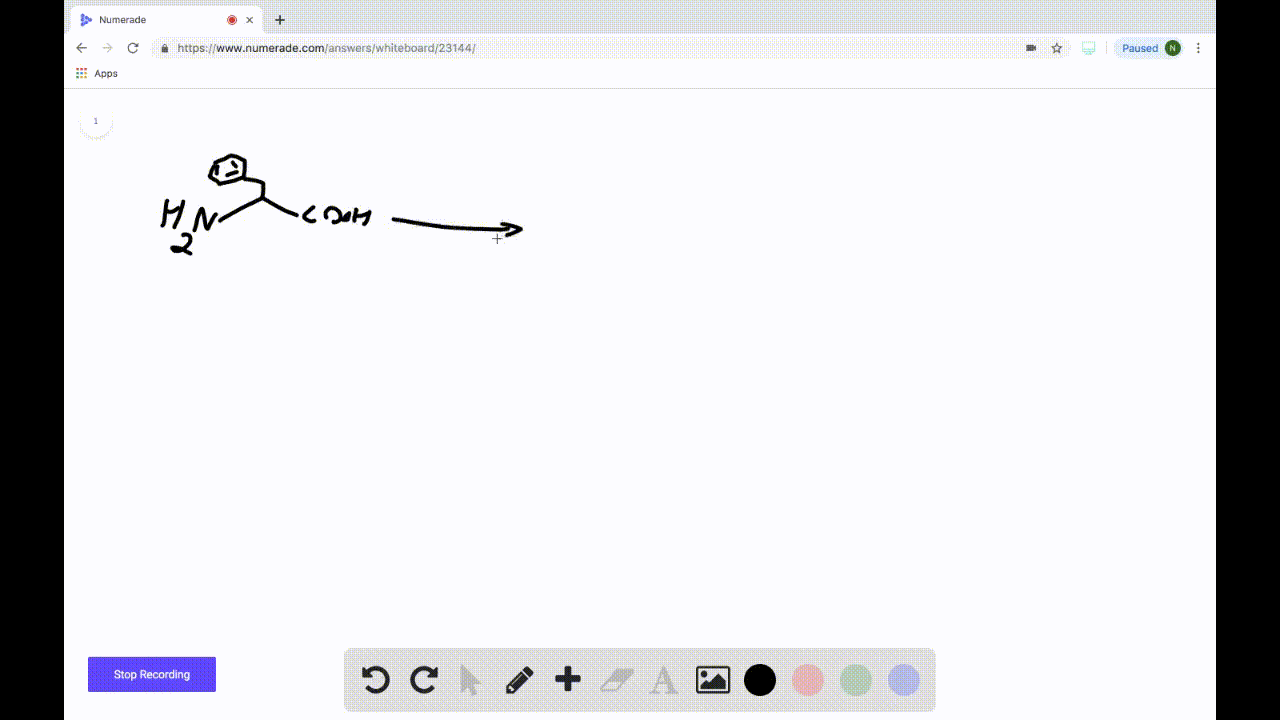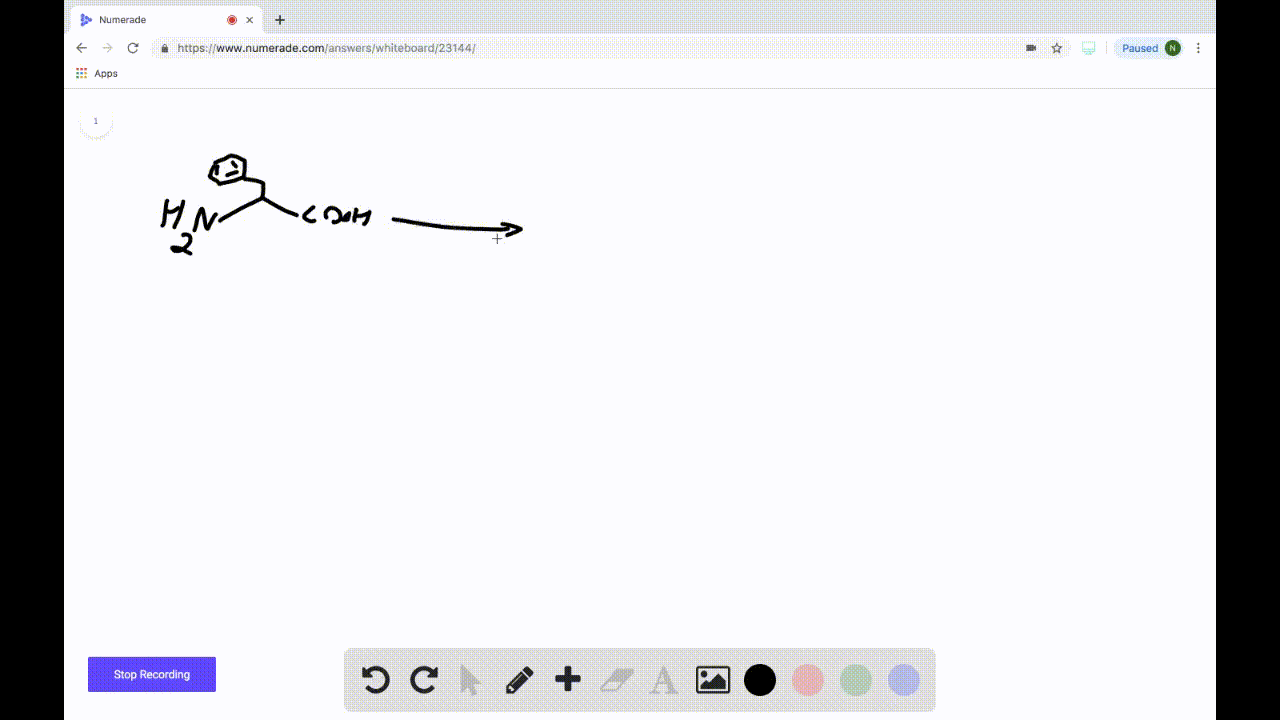
SOLVEDDraw the product formed when the following amino acid is treated
The type of salt produced depends on the. The product of neutralization is a salt and water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible.
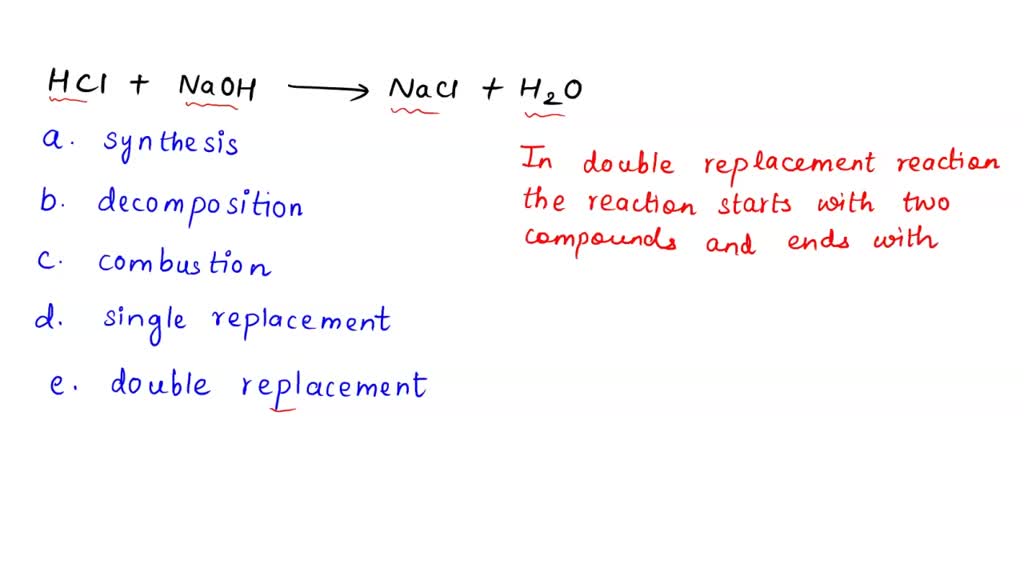
SOLVED HCl + NaOH —> NaCl + H2O What is the main classification of
The product of neutralization is a salt and water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of negligible. The type of salt produced depends on the.
A Strong Acid And A Strong Base, Such As Hcl(Aq) And Naoh(Aq) Will React To Form A Neutral Solution Since The Conjugate Partners Produced Are Of Negligible.
The type of salt produced depends on the. The product of neutralization is a salt and water.