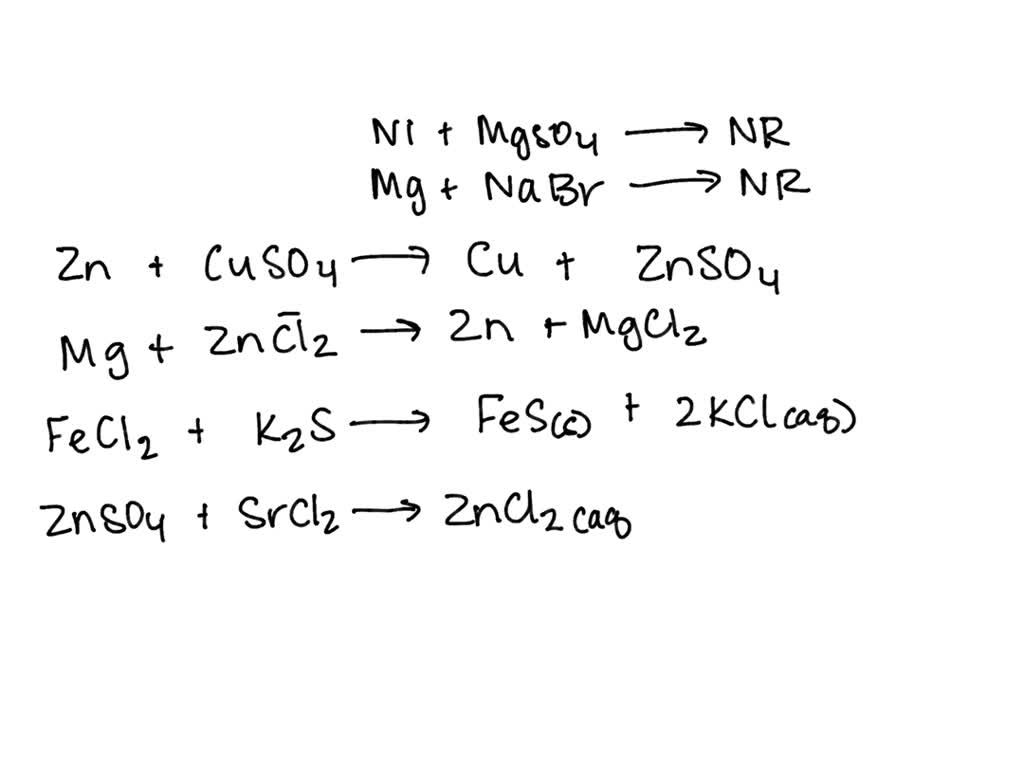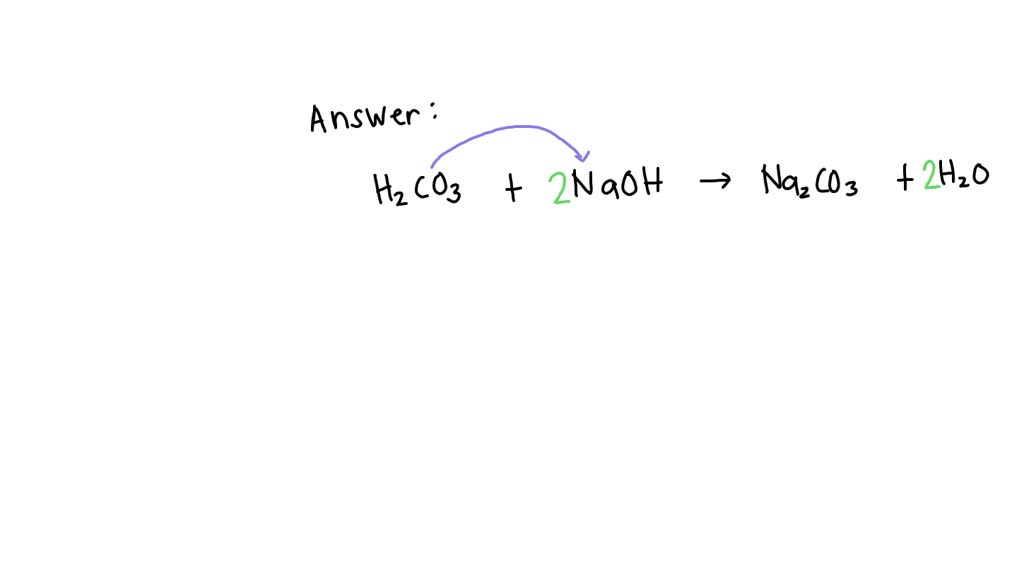What Type Of Reaction Is Na2Co3 Hcl - Na2co3 (s) + hcl (aq) = nacl (aq) + h2o (l) + co2 (g) the word equation for the reaction is: We will discuss about different characteristics of sodium carbonate and hcl acid reaction in this tutorial. In this video we determine the type of chemical reaction for the equation na2co3 + hcl = nacl + h2o + co2 (sodium carbonate +. Na 2 co 3 + hcl → nacl + co 2 + h 2 o. 2hcl +nax2cox3 2nacl +hx2o +cox2 2 h c l + n a x 2 c o x 3 2 n a c l + h x 2 o + c o x 2. This chemistry video tutorial explains how to predict the products of the reaction between sodium carbonate and hydrochloric acid. Balance the reaction of na2co3 + hcl = nacl + h2o + co2 using this chemical equation balancer!
This chemistry video tutorial explains how to predict the products of the reaction between sodium carbonate and hydrochloric acid. 2hcl +nax2cox3 2nacl +hx2o +cox2 2 h c l + n a x 2 c o x 3 2 n a c l + h x 2 o + c o x 2. In this video we determine the type of chemical reaction for the equation na2co3 + hcl = nacl + h2o + co2 (sodium carbonate +. Balance the reaction of na2co3 + hcl = nacl + h2o + co2 using this chemical equation balancer! Na2co3 (s) + hcl (aq) = nacl (aq) + h2o (l) + co2 (g) the word equation for the reaction is: Na 2 co 3 + hcl → nacl + co 2 + h 2 o. We will discuss about different characteristics of sodium carbonate and hcl acid reaction in this tutorial.
2hcl +nax2cox3 2nacl +hx2o +cox2 2 h c l + n a x 2 c o x 3 2 n a c l + h x 2 o + c o x 2. In this video we determine the type of chemical reaction for the equation na2co3 + hcl = nacl + h2o + co2 (sodium carbonate +. We will discuss about different characteristics of sodium carbonate and hcl acid reaction in this tutorial. This chemistry video tutorial explains how to predict the products of the reaction between sodium carbonate and hydrochloric acid. Na 2 co 3 + hcl → nacl + co 2 + h 2 o. Na2co3 (s) + hcl (aq) = nacl (aq) + h2o (l) + co2 (g) the word equation for the reaction is: Balance the reaction of na2co3 + hcl = nacl + h2o + co2 using this chemical equation balancer!
Sodium Carbonate to Produce Sodium Oxide and Carbon Dioxide
Na 2 co 3 + hcl → nacl + co 2 + h 2 o. 2hcl +nax2cox3 2nacl +hx2o +cox2 2 h c l + n a x 2 c o x 3 2 n a c l + h x 2 o + c o x 2. This chemistry video tutorial explains how to predict the products of the.
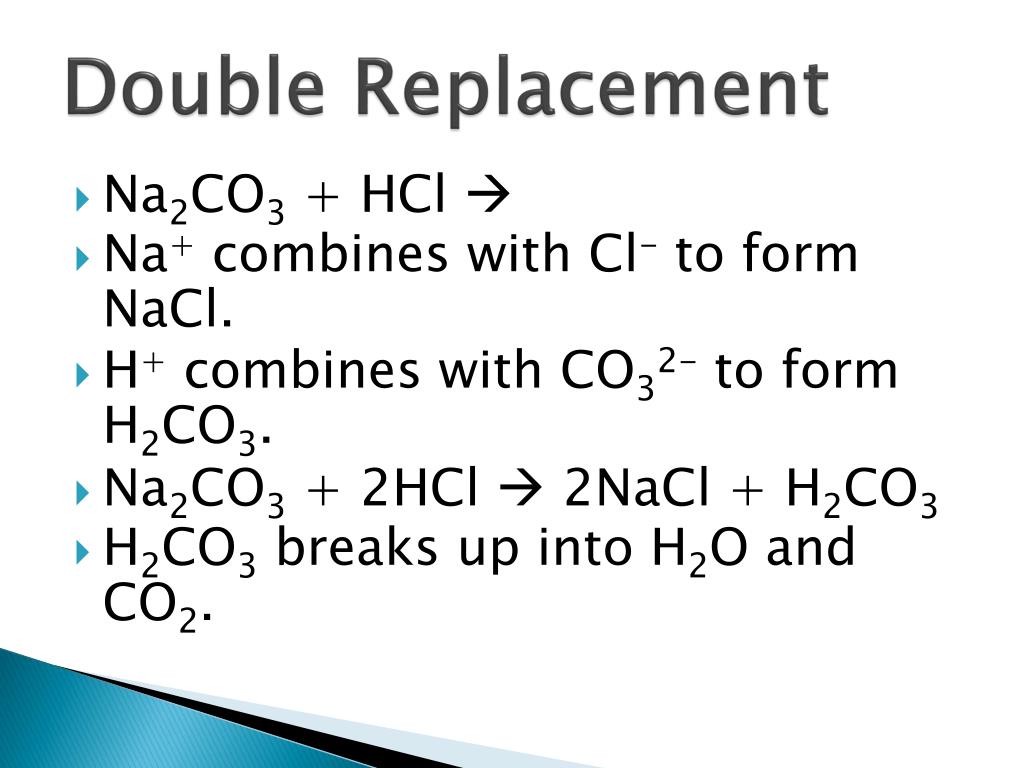
`Na_(2)CO_(3)+HCl to ………. + …….. + ………` The products in the above
2hcl +nax2cox3 2nacl +hx2o +cox2 2 h c l + n a x 2 c o x 3 2 n a c l + h x 2 o + c o x 2. Na 2 co 3 + hcl → nacl + co 2 + h 2 o. Na2co3 (s) + hcl (aq) = nacl (aq) + h2o (l) +.
SOLVED Please Complete the balance and label the raction type Complete
Balance the reaction of na2co3 + hcl = nacl + h2o + co2 using this chemical equation balancer! We will discuss about different characteristics of sodium carbonate and hcl acid reaction in this tutorial. 2hcl +nax2cox3 2nacl +hx2o +cox2 2 h c l + n a x 2 c o x 3 2 n a c l + h x.
PPT Writing Chemical Reactions PowerPoint Presentation, free download
Balance the reaction of na2co3 + hcl = nacl + h2o + co2 using this chemical equation balancer! This chemistry video tutorial explains how to predict the products of the reaction between sodium carbonate and hydrochloric acid. We will discuss about different characteristics of sodium carbonate and hcl acid reaction in this tutorial. 2hcl +nax2cox3 2nacl +hx2o +cox2 2 h.
Balanced Equation of Sodium Carbonate and Hydrochloric Acid
Na 2 co 3 + hcl → nacl + co 2 + h 2 o. We will discuss about different characteristics of sodium carbonate and hcl acid reaction in this tutorial. 2hcl +nax2cox3 2nacl +hx2o +cox2 2 h c l + n a x 2 c o x 3 2 n a c l + h x 2 o +.
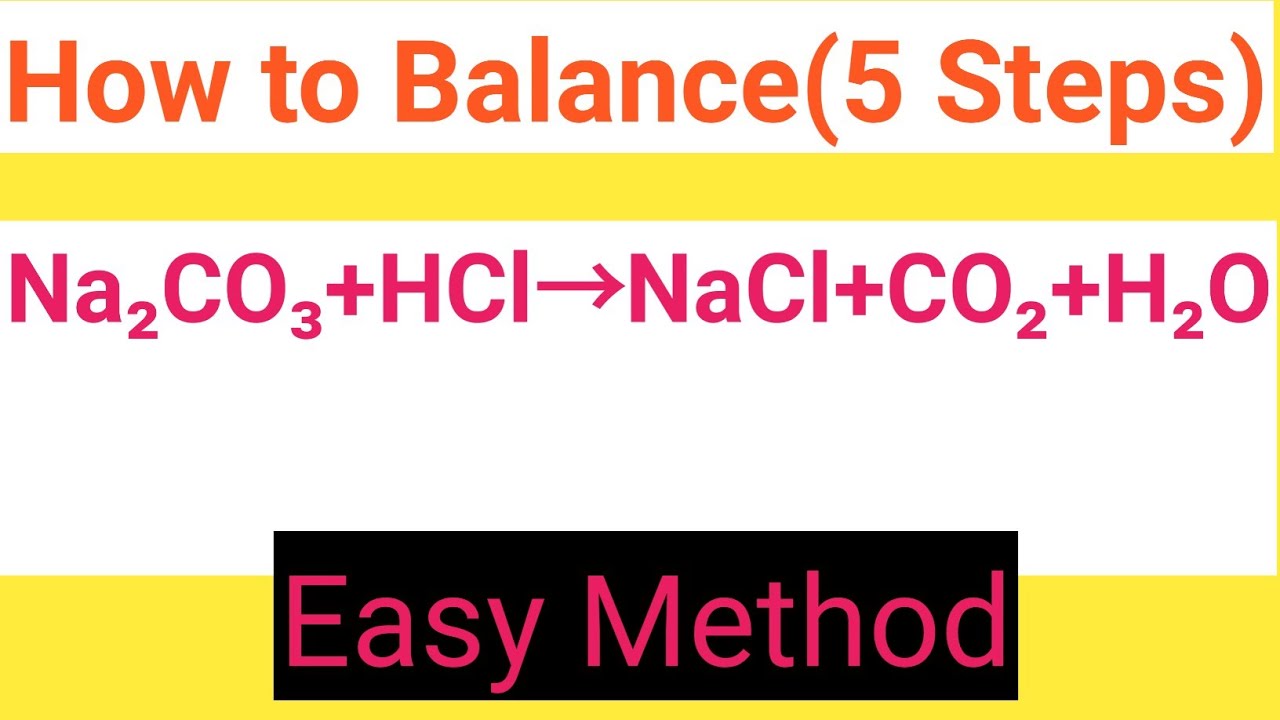
Na2CO3+HCl=NaCl+CO2+H2O Balanced EquationSodium carbonate
2hcl +nax2cox3 2nacl +hx2o +cox2 2 h c l + n a x 2 c o x 3 2 n a c l + h x 2 o + c o x 2. Na2co3 (s) + hcl (aq) = nacl (aq) + h2o (l) + co2 (g) the word equation for the reaction is: Na 2 co 3 + hcl.
SOLVED Indicates the type of reaction Na2CO3 + HCl → NaCl + H2CO3 show
Na2co3 (s) + hcl (aq) = nacl (aq) + h2o (l) + co2 (g) the word equation for the reaction is: Na 2 co 3 + hcl → nacl + co 2 + h 2 o. In this video we determine the type of chemical reaction for the equation na2co3 + hcl = nacl + h2o + co2 (sodium carbonate.
SOLVED H2CO3 + NaOH → Na2CO3 + H2O identify its type of reaction
Na 2 co 3 + hcl → nacl + co 2 + h 2 o. In this video we determine the type of chemical reaction for the equation na2co3 + hcl = nacl + h2o + co2 (sodium carbonate +. Na2co3 (s) + hcl (aq) = nacl (aq) + h2o (l) + co2 (g) the word equation for the reaction.
CHEMICAL REACTION Na2CO3 + 2HCl Unleashed
Balance the reaction of na2co3 + hcl = nacl + h2o + co2 using this chemical equation balancer! In this video we determine the type of chemical reaction for the equation na2co3 + hcl = nacl + h2o + co2 (sodium carbonate +. This chemistry video tutorial explains how to predict the products of the reaction between sodium carbonate and.
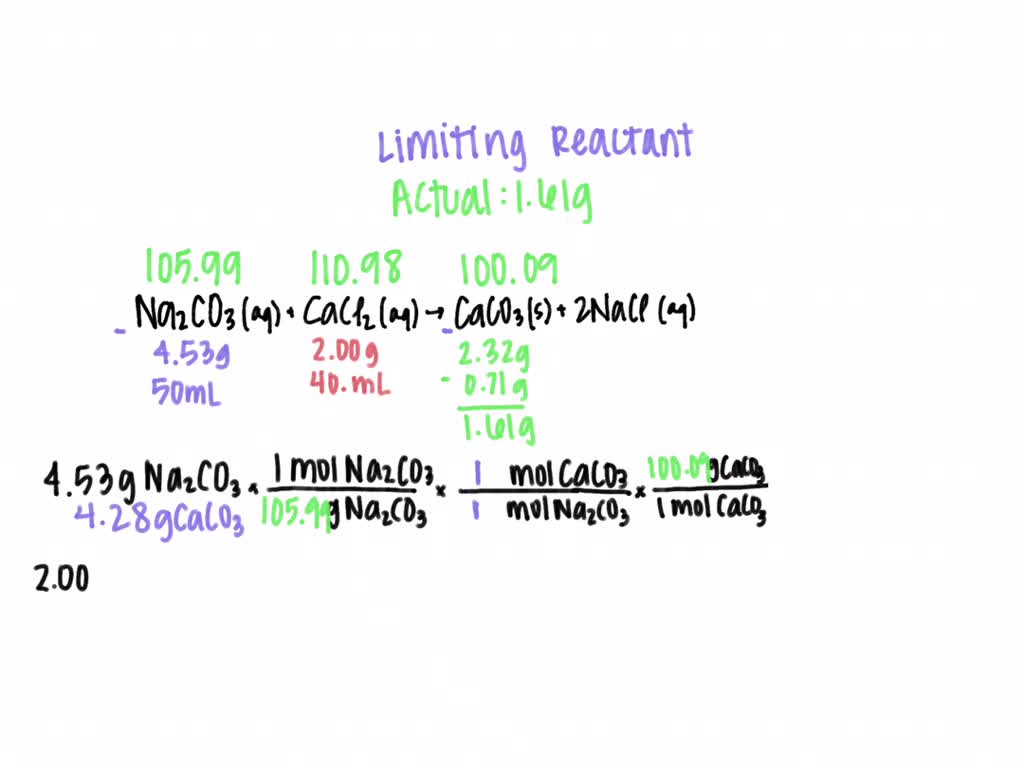
SOLVED Reaction Na2CO3(aq) + CaCl2(aq) → CaCO3(s) + 2NaCl(aq) Mass
2hcl +nax2cox3 2nacl +hx2o +cox2 2 h c l + n a x 2 c o x 3 2 n a c l + h x 2 o + c o x 2. We will discuss about different characteristics of sodium carbonate and hcl acid reaction in this tutorial. In this video we determine the type of chemical reaction for.
Na 2 Co 3 + Hcl → Nacl + Co 2 + H 2 O.
In this video we determine the type of chemical reaction for the equation na2co3 + hcl = nacl + h2o + co2 (sodium carbonate +. We will discuss about different characteristics of sodium carbonate and hcl acid reaction in this tutorial. Na2co3 (s) + hcl (aq) = nacl (aq) + h2o (l) + co2 (g) the word equation for the reaction is: 2hcl +nax2cox3 2nacl +hx2o +cox2 2 h c l + n a x 2 c o x 3 2 n a c l + h x 2 o + c o x 2.
Balance The Reaction Of Na2Co3 + Hcl = Nacl + H2O + Co2 Using This Chemical Equation Balancer!
This chemistry video tutorial explains how to predict the products of the reaction between sodium carbonate and hydrochloric acid.