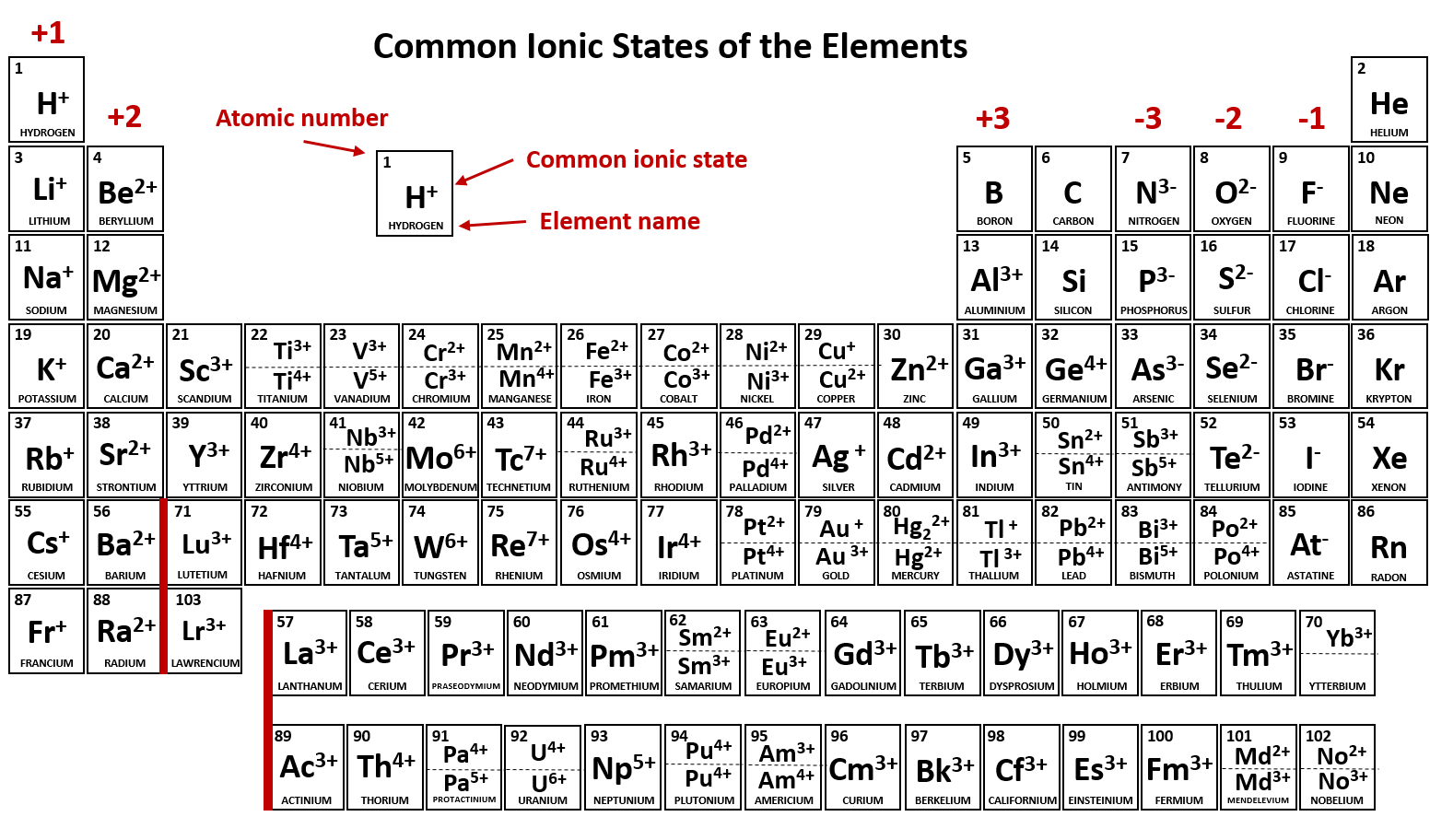
Which Ion Is This Atom Most Likely To Form - Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: 1s2 2s2 2p6 3s1 a. Which ion does it most likely form? An atom of selenium(se) forms a monatomic ion. A mercury ion with a negative 2 charge b. A potassium ion with a negative 1. Which atom is most likely to accept electrons to form an ionic bond?
Which atom is most likely to accept electrons to form an ionic bond? 1s2 2s2 2p6 3s1 a. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: A mercury ion with a negative 2 charge b. Which ion does it most likely form? An atom of selenium(se) forms a monatomic ion. A potassium ion with a negative 1.
Which atom is most likely to accept electrons to form an ionic bond? A mercury ion with a negative 2 charge b. Which ion does it most likely form? An atom of selenium(se) forms a monatomic ion. A potassium ion with a negative 1. 1s2 2s2 2p6 3s1 a. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration:
What Are the 3 Major Ions? Infrared for Health
A mercury ion with a negative 2 charge b. An atom of selenium(se) forms a monatomic ion. 1s2 2s2 2p6 3s1 a. Which atom is most likely to accept electrons to form an ionic bond? A potassium ion with a negative 1.
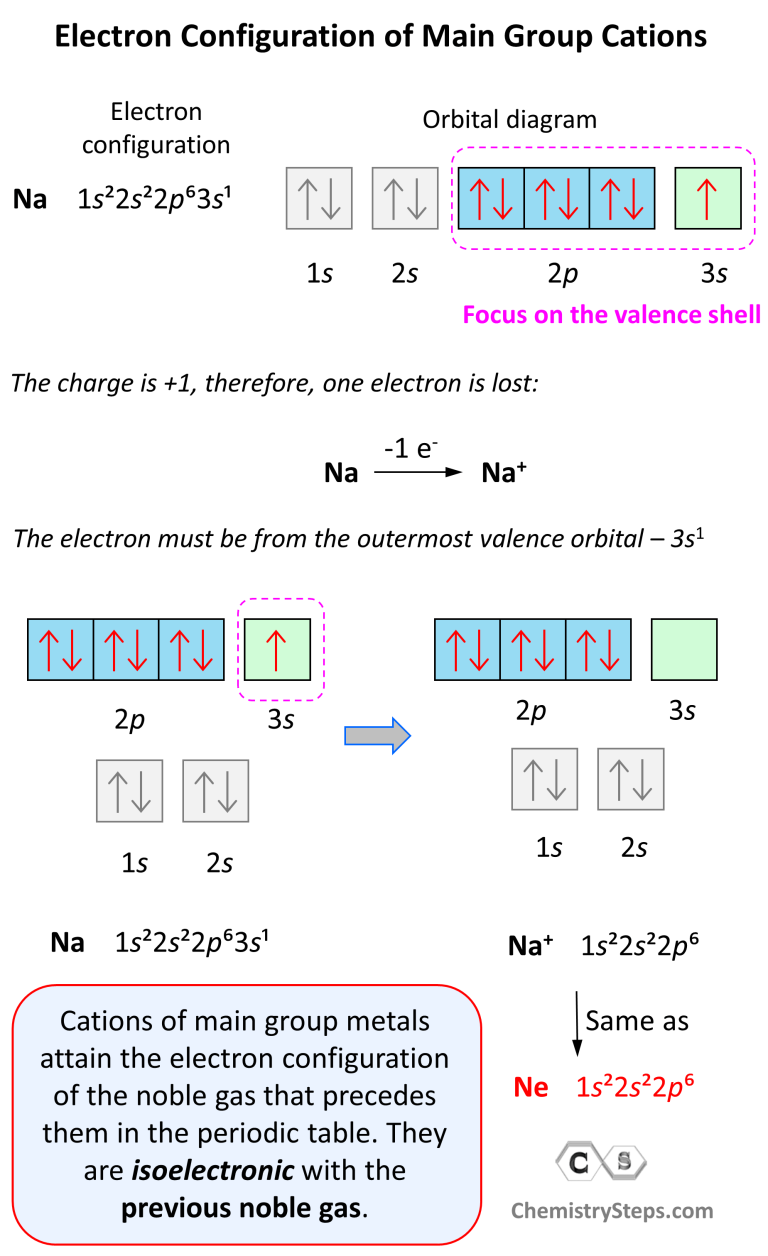
Electron Configurations of Ions Chemistry Steps
Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: Which ion does it most likely form? 1s2 2s2 2p6 3s1 a. A mercury ion with a negative 2 charge b. An atom of selenium(se) forms a monatomic ion.
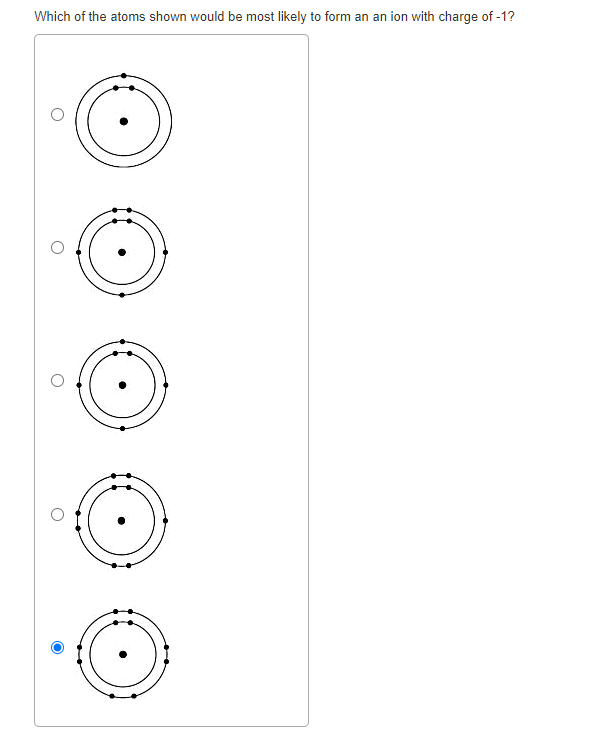
Solved Which of the atoms shown would be most likely to form
An atom of selenium(se) forms a monatomic ion. A mercury ion with a negative 2 charge b. A potassium ion with a negative 1. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: Which atom is most likely to accept electrons to form an ionic bond?
Molecular and ionic compounds Chemistry for specialties (2022)
1s2 2s2 2p6 3s1 a. A potassium ion with a negative 1. A mercury ion with a negative 2 charge b. Which atom is most likely to accept electrons to form an ionic bond? Which ion does it most likely form?
Draw the Lewis Dot Structure for the following atoms. Then determine
A potassium ion with a negative 1. Which ion does it most likely form? Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: An atom of selenium(se) forms a monatomic ion. Which atom is most likely to accept electrons to form an ionic bond?
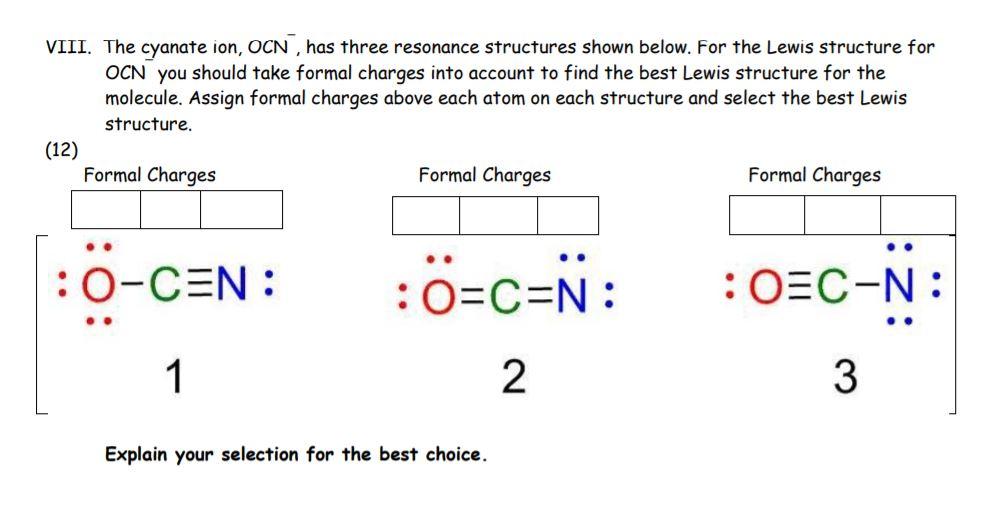
(Solved) VIII. The Cyanate Ion, OCN , Has Three Resonance Structures
A mercury ion with a negative 2 charge b. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: 1s2 2s2 2p6 3s1 a. Which ion does it most likely form? Which atom is most likely to accept electrons to form an ionic bond?
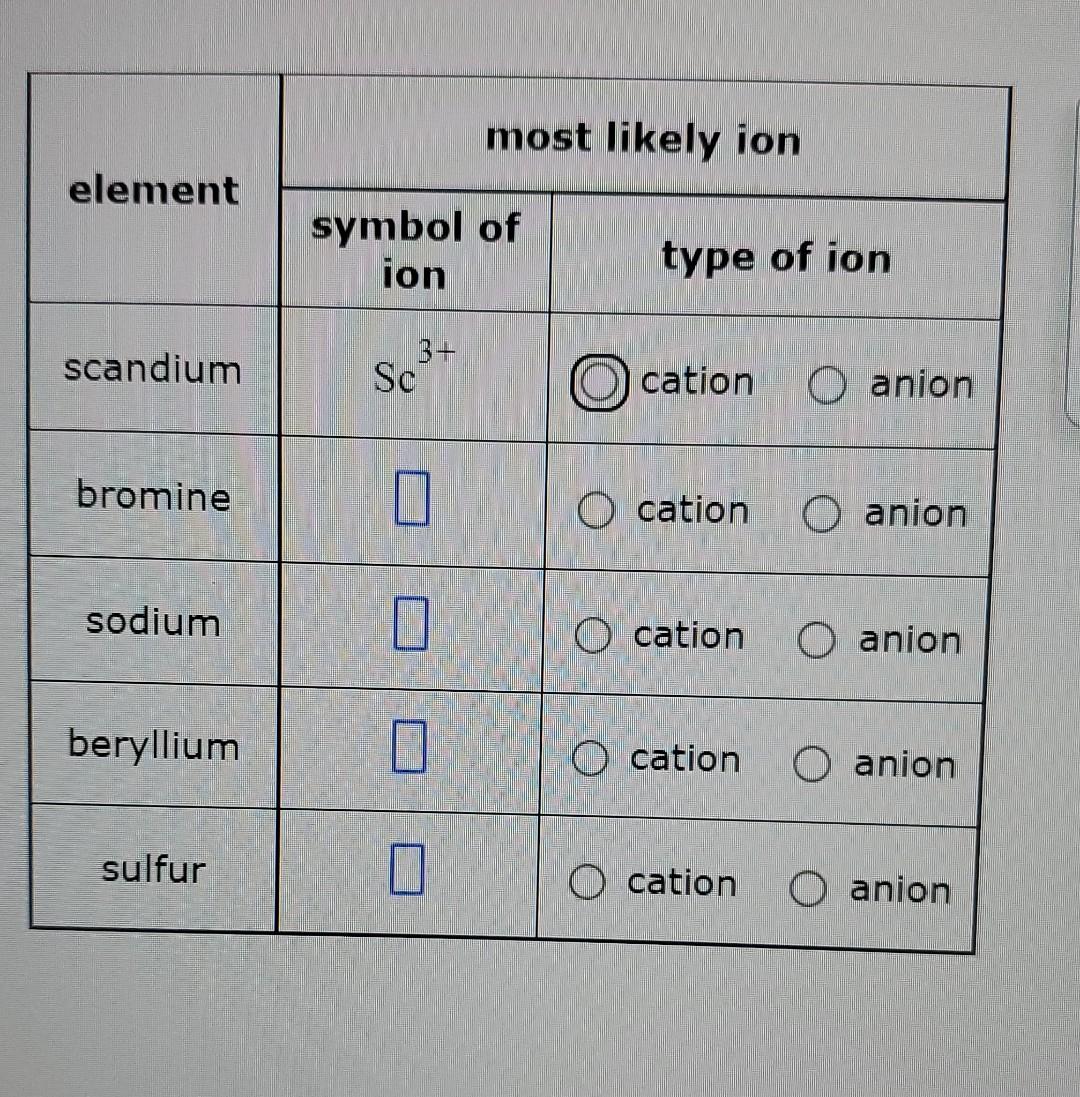
Solved most likely ion element symbol of ion type of ion
Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: Which atom is most likely to accept electrons to form an ionic bond? An atom of selenium(se) forms a monatomic ion. Which ion does it most likely form? 1s2 2s2 2p6 3s1 a.
Difference Between Atom And Ion
1s2 2s2 2p6 3s1 a. An atom of selenium(se) forms a monatomic ion. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: A potassium ion with a negative 1. Which ion does it most likely form?
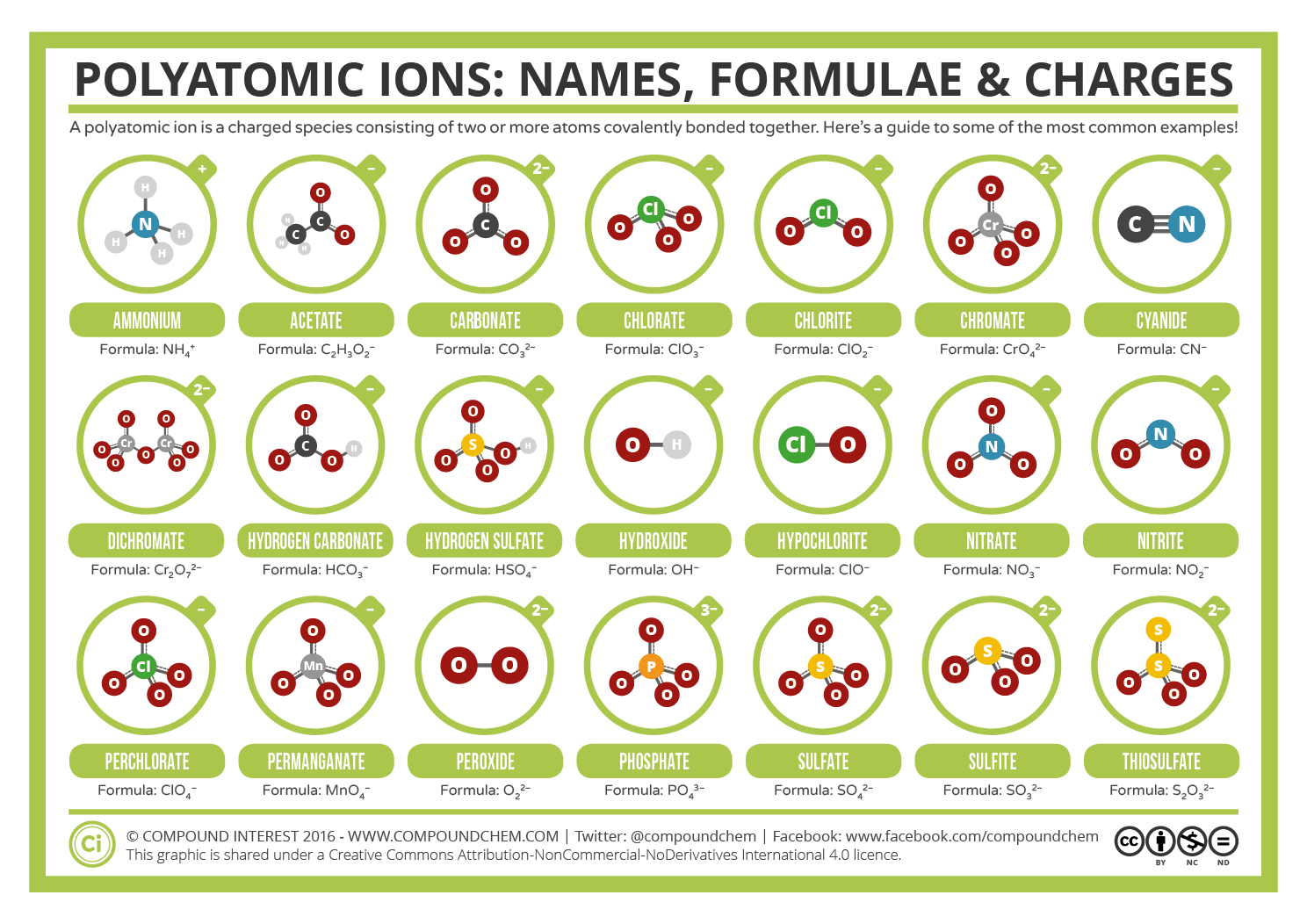
Polyatomic Ions Naming and Formulas Study Guide Inspirit
Which atom is most likely to accept electrons to form an ionic bond? A potassium ion with a negative 1. A mercury ion with a negative 2 charge b. Which ion does it most likely form? 1s2 2s2 2p6 3s1 a.
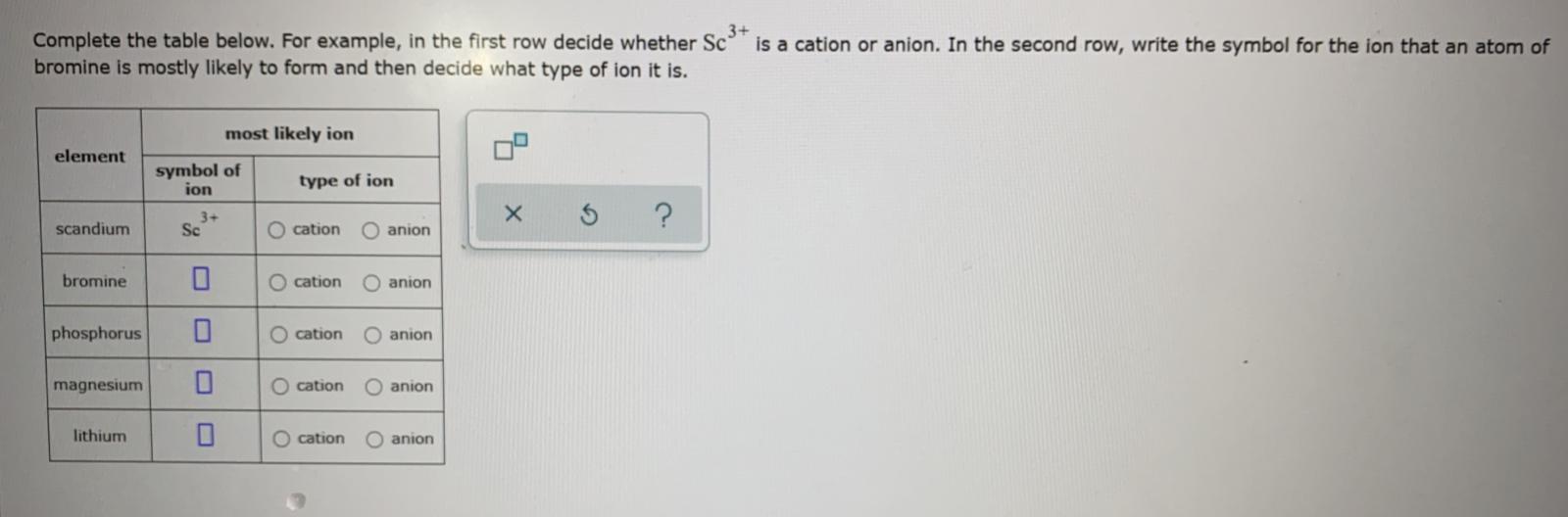
Solved Complete the table below. For example, in the first
Which atom is most likely to accept electrons to form an ionic bond? 1s2 2s2 2p6 3s1 a. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: An atom of selenium(se) forms a monatomic ion. A mercury ion with a negative 2 charge b.
1S2 2S2 2P6 3S1 A.
An atom of selenium(se) forms a monatomic ion. A mercury ion with a negative 2 charge b. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: A potassium ion with a negative 1.
Which Atom Is Most Likely To Accept Electrons To Form An Ionic Bond?
Which ion does it most likely form?